Investigating the Cellular Specificity in Tumors of a Surface-Converting Nanoparticle by Multimodal Imaging
- PMID: 28316241
- PMCID: PMC5567755
- DOI: 10.1021/acs.bioconjchem.7b00086
Investigating the Cellular Specificity in Tumors of a Surface-Converting Nanoparticle by Multimodal Imaging
Abstract
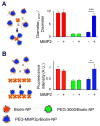
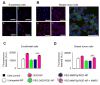
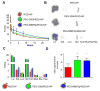
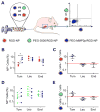
Active targeting of nanoparticles through surface functionalization is a common strategy to enhance tumor delivery specificity. However, active targeting strategies tend to work against long polyethylene glycol's shielding effectiveness and associated favorable pharmacokinetics. To overcome these limitations, we developed a matrix metalloproteinase-2 sensitive surface-converting polyethylene glycol coating. This coating prevents nanoparticle-cell interaction in the bloodstream, but, once exposed to matrix metalloproteinase-2, i.e., when the nanoparticles accumulate within the tumor interstitium, the converting polyethylene glycol coating is cleaved, and targeting ligands become available for binding to tumor cells. In this study, we applied a comprehensive multimodal imaging strategy involving optical, nuclear, and magnetic resonance imaging methods to evaluate this coating approach in a breast tumor mouse model. The data obtained revealed that this surface-converting coating enhances the nanoparticle's blood half-life and tumor accumulation and ultimately results in improved tumor-cell targeting. Our results show that this enzyme-specific surface-converting coating ensures a high cell-targeting specificity without compromising favorable nanoparticle pharmacokinetics.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


Similar articles
-
Preferential tumor accumulation and desirable interstitial penetration of poly(lactic-co-glycolic acid) nanoparticles with dual coating of chitosan oligosaccharide and polyethylene glycol-poly(D,L-lactic acid).Acta Biomater. 2016 Jan;29:248-260. doi: 10.1016/j.actbio.2015.10.017. Epub 2015 Oct 22. Acta Biomater. 2016. PMID: 26476340
-
Amphiphilic Polymer-Modified Uniform CuFeSe2 Nanoparticles for CT/MR Dual-Modal Imaging.Contrast Media Mol Imaging. 2020 Dec 30;2020:4891325. doi: 10.1155/2020/4891325. eCollection 2020. Contrast Media Mol Imaging. 2020. PMID: 33456402 Free PMC article.
-
Synthesis of Lipophilic Core-Shell Fe3O4@SiO2@Au Nanoparticles and Polymeric Entrapment into Nanomicelles: A Novel Nanosystem for in Vivo Active Targeting and Magnetic Resonance-Photoacoustic Dual Imaging.Bioconjug Chem. 2017 May 17;28(5):1382-1390. doi: 10.1021/acs.bioconjchem.7b00076. Epub 2017 May 3. Bioconjug Chem. 2017. PMID: 28453929
-
Multifunctional nanoparticles as nanocarrier for vincristine sulfate delivery to overcome tumor multidrug resistance.Mol Pharm. 2014 Mar 3;11(3):885-94. doi: 10.1021/mp400547u. Epub 2014 Jan 31. Mol Pharm. 2014. PMID: 24483832
-
Shortwave infrared emitting multicolored nanoprobes for biomarker-specific cancer imaging in vivo.BMC Cancer. 2020 Nov 10;20(1):1082. doi: 10.1186/s12885-020-07604-8. BMC Cancer. 2020. PMID: 33172421 Free PMC article.
Cited by
-
Microglia and metastases to the central nervous system: victim, ravager, or something else?J Exp Clin Cancer Res. 2022 Nov 21;41(1):327. doi: 10.1186/s13046-022-02535-7. J Exp Clin Cancer Res. 2022. PMID: 36411434 Free PMC article. Review.
-
[Anti-CD206 antibody-conjugated Fe3O4-based PLGA nanoparticles selectively promotes M1 polarization of tumorassociated macrophages in mice].Nan Fang Yi Ke Da Xue Xue Bao. 2020 Feb 29;40(2):246-254. doi: 10.12122/j.issn.1673-4254.2020.02.17. Nan Fang Yi Ke Da Xue Xue Bao. 2020. PMID: 32376536 Free PMC article. Chinese.
-
Transformative hyaluronic acid-based active targeting supramolecular nanoplatform improves long circulation and enhances cellular uptake in cancer therapy.Acta Pharm Sin B. 2019 Mar;9(2):397-409. doi: 10.1016/j.apsb.2018.11.006. Epub 2018 Nov 29. Acta Pharm Sin B. 2019. PMID: 30972285 Free PMC article.
-
Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine.Molecules. 2024 May 31;29(11):2584. doi: 10.3390/molecules29112584. Molecules. 2024. PMID: 38893460 Free PMC article. Review.
-
Matrix Metalloproteinase-9-Responsive Nanogels for Proximal Surface Conversion and Activated Cellular Uptake.Biomacromolecules. 2018 Mar 12;19(3):860-871. doi: 10.1021/acs.biomac.7b01659. Epub 2018 Feb 8. Biomacromolecules. 2018. PMID: 29360342 Free PMC article.
References
-
- Hrkach J, Von Hoff D, Mukkaram Ali M, Andrianova E, Auer J, Campbell T, De Witt D, Figa M, Figueiredo M, Horhota A, et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharm \acological profile. Sci Transl Med. 2012;4:128ra39. - PubMed
-
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

