Methylation of FOXP3 TSDR Underlies the Impaired Suppressive Function of Tregs from Long-term Belatacept-Treated Kidney Transplant Patients
- PMID: 28316600
- PMCID: PMC5334349
- DOI: 10.3389/fimmu.2017.00219
Methylation of FOXP3 TSDR Underlies the Impaired Suppressive Function of Tregs from Long-term Belatacept-Treated Kidney Transplant Patients
Abstract
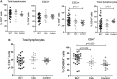
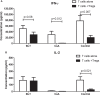
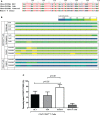
Regulatory T cells (Tregs) are considered key players in the prevention of allograft rejection in transplanted patients. Belatacept (BLT) is an effective alternative to calcineurin inhibitors that appears to preserve graft survival and function; however, the impact of this drug in the homeostasis of Tregs in transplanted patients remains controversial. Here, we analyzed the phenotype, function, and the epigenetic status of the Treg-specific demethylated region (TSDR) in FOXP3 of circulating Tregs from long-term kidney transplant patients under BLT or Cyclosporine A treatment. We found a significant reduction in the proportion of CD4+CD25hiCD127lo/-FOXP3+ T cells in all patients compared to healthy individual (controls). Interestingly, only BLT-treated patients displayed an enrichment of the CD45RA+ "naïve" Tregs, while the expression of Helios, a marker used to identify stable FOXP3+ thymic Tregs remained unaffected. Functional analysis demonstrated that Tregs from transplanted patients displayed a significant reduction in their suppressive capacity compared to Tregs from controls, which is associated with decreased levels of FOXP3 and CD25. Analysis of the methylation status of the FOXP3 gene showed that BLT treatment results in methylation of CpG islands within the TSDR, which could be associated with the impaired Treg suppression function. Our data indicate that analysis of circulating Tregs cannot be used as a marker for assessing tolerance toward the allograft in long-term kidney transplant patients. Trial registration number IM103008.
Keywords: Cyclosporine A; Treg; belatacept; suppression; tolerance; transplantation.
Figures





References
-
- Vincenti F, Charpentier B, Vanrenterghem Y, Rostaing L, Bresnahan B, Darji P, et al. A phase III study of belatacept-based immunosuppression regimens versus cyclosporine in renal transplant recipients (BENEFIT study). Am J Transplant (2010) 10(3):535–46. 10.1111/j.1600-6143.2009.03005.x - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

