Guarana (Paullinia cupana Mart.) attenuates methylmercury-induced toxicity in Caenorhabditis elegans
- PMID: 28316775
- PMCID: PMC5354468
- DOI: 10.1039/C6TX00161K
Guarana (Paullinia cupana Mart.) attenuates methylmercury-induced toxicity in Caenorhabditis elegans
Abstract
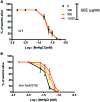
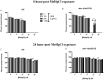
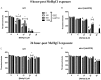
The influence of routine guarana (Paullinia cupana) consumption on apparent tolerance to mercury intoxication has been proposed. The present study investigated this hypothesis in Caenorhabditis elegans, a suitable experimental model for studies in toxicology. Wild type (WT) and skn-1 (ok2315) worm strains were pretreated with guarana ethanolic extract (GEE) from larvae 1 (L1) to L4 stage and then exposed for 6 hours to methylmercury (MeHg). The analyses included evaluation of GEE's effects on lethality, developmental delay, feeding, locomotion, gene expression (sod-3, gst-4, sir-2.1, hsf-1, snn-1, mtl-1, mtl-2, aat-1, aat-2 and aat-3) and antioxidant activity. GEE pre-treatment had no aberrant effects on WT worms exposed to MeHg, and protected skn-1 (ok2315) worms, which are more susceptible to environmental stresses. Protective effects of GEE might be dependent on modulation of genes other than those directly involved in antioxidant activity. GEE increased the expression of genes involved in metal transport (aat-2), metal detoxification (mtl-1 and mtl-2) and antioxidant responses (sir-2.1 and sod-3). Thus, routine consumption of guarana might be beneficial in protecting against MeHg-induced toxicity.
Keywords: mercury; natural products; neurological disorders; xanthines.
Conflict of interest statement
Conflicts of interest There are no conflicts of interest to declare.
Figures


Similar articles
-
Guarana improves behavior and inflammatory alterations triggered by methylmercury exposure: an in vivo fruit fly and in vitro neural cells study.Environ Sci Pollut Res Int. 2019 May;26(15):15069-15083. doi: 10.1007/s11356-019-04881-0. Epub 2019 Mar 27. Environ Sci Pollut Res Int. 2019. PMID: 30915696
-
Mechanisms involved in anti-aging effects of guarana (Paullinia cupana) in Caenorhabditis elegans.Braz J Med Biol Res. 2018 Jul 2;51(9):e7552. doi: 10.1590/1414-431X20187552. Braz J Med Biol Res. 2018. PMID: 29972429 Free PMC article.
-
Guarana (Paullinia cupana Mart.) protects against amyloid-β toxicity in Caenorhabditis elegans through heat shock protein response activation.Nutr Neurosci. 2020 Jun;23(6):444-454. doi: 10.1080/1028415X.2018.1517473. Epub 2018 Sep 9. Nutr Neurosci. 2020. PMID: 30198423
-
Effects of the consumption of guarana on human health: A narrative review.Compr Rev Food Sci Food Saf. 2022 Jan;21(1):272-295. doi: 10.1111/1541-4337.12862. Epub 2021 Nov 9. Compr Rev Food Sci Food Saf. 2022. PMID: 34755935 Review.
-
Guarana: revisiting a highly caffeinated plant from the Amazon.J Ethnopharmacol. 2013 Oct 28;150(1):14-31. doi: 10.1016/j.jep.2013.08.023. Epub 2013 Aug 24. J Ethnopharmacol. 2013. PMID: 23981847 Review.
Cited by
-
Methylmercury Induces Metabolic Alterations in Caenorhabditis elegans: Role for C/EBP Transcription Factor.Toxicol Sci. 2020 Mar 1;174(1):112-123. doi: 10.1093/toxsci/kfz244. Toxicol Sci. 2020. PMID: 31851340 Free PMC article.
-
Protective Effects of Plathymenia reticulata and Connarus favosus Aqueous Extracts against Cadmium- and Mercury-Induced Toxicities.Toxicol Res. 2019 Jan;35(1):25-35. doi: 10.5487/TR.2019.35.1.025. Epub 2018 Jan 15. Toxicol Res. 2019. PMID: 30766655 Free PMC article.
-
Genetic factors in methylmercury-induced neurotoxicity: What have we learned from Caenorhabditis elegans models?Adv Neurotoxicol. 2023;9:271-290. doi: 10.1016/bs.ant.2023.01.006. Epub 2023 Mar 13. Adv Neurotoxicol. 2023. PMID: 37389202 Free PMC article. No abstract available.
-
Therapeutic potential of traditional herbal plants and their polyphenols in alleviation of mercury toxicity.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jul;398(7):7737-7763. doi: 10.1007/s00210-025-03807-7. Epub 2025 Feb 6. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39912903 Review.
-
Guarana improves behavior and inflammatory alterations triggered by methylmercury exposure: an in vivo fruit fly and in vitro neural cells study.Environ Sci Pollut Res Int. 2019 May;26(15):15069-15083. doi: 10.1007/s11356-019-04881-0. Epub 2019 Mar 27. Environ Sci Pollut Res Int. 2019. PMID: 30915696
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

