Polymeric mechanical amplifiers of immune cytokine-mediated apoptosis
- PMID: 28317839
- PMCID: PMC5364380
- DOI: 10.1038/ncomms14179
Polymeric mechanical amplifiers of immune cytokine-mediated apoptosis
Abstract
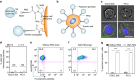
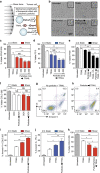
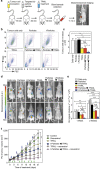
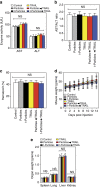
Physical forces affect tumour growth, progression and metastasis. Here, we develop polymeric mechanical amplifiers that exploit in vitro and in vivo physical forces to increase immune cytokine-mediated tumour cell apoptosis. Mechanical amplifiers, consisting of biodegradable polymeric particles tethered to the tumour cell surface via polyethylene glycol linkers, increase the apoptotic effect of an immune cytokine on tumour cells under fluid shear exposure by as much as 50% compared with treatment under static conditions. We show that targeted polymeric particles delivered to tumour cells in vivo amplify the apoptotic effect of a subsequent treatment of immune cytokine, reduce circulating tumour cells in blood and overall tumour cell burden by over 90% and reduce solid tumour growth in combination with the antioxidant resveratrol. The work introduces a potentially new application for a broad range of micro- and nanoparticles to maximize receptor-mediated signalling and function in the presence of physical forces.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


Similar articles
-
The roadmap of TRAIL apoptotic pathway-targeted cancer therapies: What is next?Expert Rev Anticancer Ther. 2012 May;12(5):547-9. doi: 10.1586/era.12.33. Expert Rev Anticancer Ther. 2012. PMID: 22594889 No abstract available.
-
Evaluation of preventive and therapeutic activity of novel non-steroidal anti-inflammatory drug, CG100649, in colon cancer: Increased expression of TNF-related apoptosis-inducing ligand receptors enhance the apoptotic response to combination treatment with TRAIL.Oncol Rep. 2015 Apr;33(4):1947-55. doi: 10.3892/or.2015.3793. Epub 2015 Feb 10. Oncol Rep. 2015. PMID: 25672292
-
Combination Nanopreparations of a Novel Proapoptotic Drug - NCL-240, TRAIL and siRNA.Pharm Res. 2016 Jul;33(7):1587-601. doi: 10.1007/s11095-016-1899-z. Epub 2016 Mar 7. Pharm Res. 2016. PMID: 26951567
-
Bortezomib and TRAIL: a perfect match for apoptotic elimination of tumour cells?Crit Rev Oncol Hematol. 2013 Mar;85(3):363-72. doi: 10.1016/j.critrevonc.2012.08.001. Epub 2012 Sep 1. Crit Rev Oncol Hematol. 2013. PMID: 22944363 Review.
-
TRAIL receptor signalling and modulation: Are we on the right TRAIL?Cancer Treat Rev. 2009 May;35(3):280-8. doi: 10.1016/j.ctrv.2008.11.006. Epub 2008 Dec 30. Cancer Treat Rev. 2009. PMID: 19117685 Review.
Cited by
-
Drug-free albumin-triggered sensitization of cancer cells to anticancer drugs.J Control Release. 2019 Jan 10;293:84-93. doi: 10.1016/j.jconrel.2018.11.015. Epub 2018 Nov 19. J Control Release. 2019. PMID: 30465822 Free PMC article.
-
Acoustic technologies for the orchestration of cellular functions for therapeutic applications.Sci Adv. 2025 Jul 18;11(29):eadu4759. doi: 10.1126/sciadv.adu4759. Epub 2025 Jul 18. Sci Adv. 2025. PMID: 40680134 Free PMC article. Review.
-
Nanoparticles for Immune Cytokine TRAIL-Based Cancer Therapy.ACS Nano. 2018 Feb 27;12(2):912-931. doi: 10.1021/acsnano.7b05876. Epub 2018 Feb 6. ACS Nano. 2018. PMID: 29378114 Free PMC article. Review.
-
Light-triggered molecular mechanotherapy of tumor using membrane-mimicking conjugated oligoelectrolytes.Sci Adv. 2025 Aug 22;11(34):eady3349. doi: 10.1126/sciadv.ady3349. Epub 2025 Aug 22. Sci Adv. 2025. PMID: 40845106 Free PMC article.
-
Lipid Phase Separation in Vesicles Enhances TRAIL-Mediated Cytotoxicity.Nano Lett. 2022 Apr 13;22(7):2627-2634. doi: 10.1021/acs.nanolett.1c04365. Epub 2022 Mar 17. Nano Lett. 2022. PMID: 35298184 Free PMC article.
References
-
- Stevens M. M. & George J. H. Exploring and engineering the cell surface interface. Science 310, 1135–1138 (2005). - PubMed
-
- Mannix R. J. et al.. Nanomagnetic actuation of receptor-mediated signal transduction. Nat. Nanotechnol. 3, 36–40 (2007). - PubMed
-
- Lee J.-H. et al.. Artificial control of cell signaling and growth by magnetic nanoparticles. Angew. Chem. Int. Ed. 49, 5698–5702 (2010). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

