Cyclooxygenase-2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents
- PMID: 28317866
- PMCID: PMC5357798
- DOI: 10.1038/srep44701
Cyclooxygenase-2 facilitates dengue virus replication and serves as a potential target for developing antiviral agents
Abstract
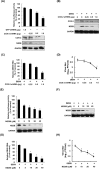
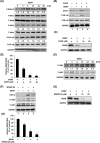
Cyclooxygenase-2 (COX-2) is one of the important mediators of inflammation in response to viral infection, and it contributes to viral replication, for example, cytomegalovirus or hepatitis C virus replication. The role of COX-2 in dengue virus (DENV) replication remains unclear. In the present study, we observed an increased level of COX-2 in patients with dengue fever compared with healthy donors. Consistent with the clinical data, an elevated level of COX-2 expression was also observed in DENV-infected ICR suckling mice. Using cell-based experiments, we revealed that DENV-2 infection significantly induced COX-2 expression and prostaglandin E2 (PGE2) production in human hepatoma Huh-7 cells. The exogenous expression of COX-2 or PGE2 treatment dose-dependently enhanced DENV-2 replication. In contrast, COX-2 gene silencing and catalytic inhibition sufficiently suppressed DENV-2 replication. In an ICR suckling mouse model, we identified that the COX-2 inhibitor NS398 protected mice from succumbing to life-threatening DENV-2 infection. By using COX-2 promoter-based analysis and specific inhibitors against signaling molecules, we identified that NF-κB and MAPK/JNK are critical factors for DENV-2-induced COX-2 expression and viral replication. Altogether, our results reveal that COX-2 is an important factor for DENV replication and can serve as a potential target for developing therapeutic agents against DENV infection.
Conflict of interest statement
The authors declare no competing financial interests.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

