Parietal operculum and motor cortex activities predict motor recovery in moderate to severe stroke
- PMID: 28317947
- PMCID: PMC5342999
- DOI: 10.1016/j.nicl.2017.01.023
Parietal operculum and motor cortex activities predict motor recovery in moderate to severe stroke
Abstract
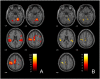
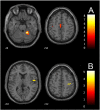
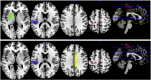
While motor recovery following mild stroke has been extensively studied with neuroimaging, mechanisms of recovery after moderate to severe strokes of the types that are often the focus for novel restorative therapies remain obscure. We used fMRI to: 1) characterize reorganization occurring after moderate to severe subacute stroke, 2) identify brain regions associated with motor recovery and 3) to test whether brain activity associated with passive movement measured in the subacute period could predict motor outcome six months later. Because many patients with large strokes involving sensorimotor regions cannot engage in voluntary movement, we used passive flexion-extension of the paretic wrist to compare 21 patients with subacute ischemic stroke to 24 healthy controls one month after stroke. Clinical motor outcome was assessed with Fugl-Meyer motor scores (motor-FMS) six months later. Multiple regression, with predictors including baseline (one-month) motor-FMS and sensorimotor network regional activity (ROI) measures, was used to determine optimal variable selection for motor outcome prediction. Sensorimotor network ROIs were derived from a meta-analysis of arm voluntary movement tasks. Bootstrapping with 1000 replications was used for internal model validation. During passive movement, both control and patient groups exhibited activity increases in multiple bilateral sensorimotor network regions, including the primary motor (MI), premotor and supplementary motor areas (SMA), cerebellar cortex, putamen, thalamus, insula, Brodmann area (BA) 44 and parietal operculum (OP1-OP4). Compared to controls, patients showed: 1) lower task-related activity in ipsilesional MI, SMA and contralesional cerebellum (lobules V-VI) and 2) higher activity in contralesional MI, superior temporal gyrus and OP1-OP4. Using multiple regression, we found that the combination of baseline motor-FMS, activity in ipsilesional MI (BA4a), putamen and ipsilesional OP1 predicted motor outcome measured 6 months later (adjusted-R2 = 0.85; bootstrap p < 0.001). Baseline motor-FMS alone predicted only 54% of the variance. When baseline motor-FMS was removed, the combination of increased activity in ipsilesional MI-BA4a, ipsilesional thalamus, contralesional mid-cingulum, contralesional OP4 and decreased activity in ipsilesional OP1, predicted better motor outcome (djusted-R2 = 0.96; bootstrap p < 0.001). In subacute stroke, fMRI brain activity related to passive movement measured in a sensorimotor network defined by activity during voluntary movement predicted motor recovery better than baseline motor-FMS alone. Furthermore, fMRI sensorimotor network activity measures considered alone allowed excellent clinical recovery prediction and may provide reliable biomarkers for assessing new therapies in clinical trial contexts. Our findings suggest that neural reorganization related to motor recovery from moderate to severe stroke results from balanced changes in ipsilesional MI (BA4a) and a set of phylogenetically more archaic sensorimotor regions in the ventral sensorimotor trend, in which OP1 and OP4 processes may complement the ipsilesional dorsal motor cortex in achieving compensatory sensorimotor recovery.
Figures



References
-
- Agnew J.A., Zeffiro T.A., Eden G.F. Left hemisphere specialization for the control of voluntary movement rate. NeuroImage. 2004;22:289–303. - PubMed
-
- Blatow M. Clinical functional MRI of sensorimotor cortex using passive motor and sensory stimulation at 3 tesla. J. Magn. Reson. Imaging. 2011;34:429–437. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

