Deoxycytidine and Deoxythymidine Treatment for Thymidine Kinase 2 Deficiency
- PMID: 28318037
- PMCID: PMC5926768
- DOI: 10.1002/ana.24922
Deoxycytidine and Deoxythymidine Treatment for Thymidine Kinase 2 Deficiency
Abstract
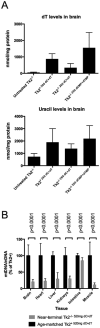
Objective: Thymidine kinase 2 (TK2), a critical enzyme in the mitochondrial pyrimidine salvage pathway, is essential for mitochondrial DNA (mtDNA) maintenance. Mutations in the nuclear gene, TK2, cause TK2 deficiency, which manifests predominantly in children as myopathy with mtDNA depletion. Molecular bypass therapy with the TK2 products, deoxycytidine monophosphate (dCMP) and deoxythymidine monophosphate (dTMP), prolongs the life span of Tk2-deficient (Tk2-/- ) mice by 2- to 3-fold. Because we observed rapid catabolism of the deoxynucleoside monophosphates to deoxythymidine (dT) and deoxycytidine (dC), we hypothesized that: (1) deoxynucleosides might be the major active agents and (2) inhibition of deoxycytidine deamination might enhance dTMP+dCMP therapy.
Methods: To test these hypotheses, we assessed two therapies in Tk2-/- mice: (1) dT+dC and (2) coadministration of the deaminase inhibitor, tetrahydrouridine (THU), with dTMP+dCMP.
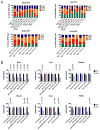
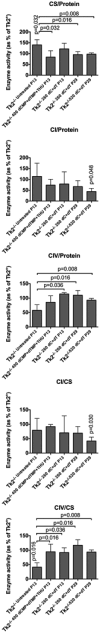
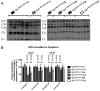
Results: We observed that dC+dT delayed disease onset, prolonged life span of Tk2-deficient mice and restored mtDNA copy number as well as respiratory chain enzyme activities and levels. In contrast, dCMP+dTMP+THU therapy decreased life span of Tk2-/- animals compared to dCMP+dTMP.
Interpretation: Our studies demonstrate that deoxynucleoside substrate enhancement is a novel therapy, which may ameliorate TK2 deficiency in patients. Ann Neurol 2017;81:641-652.
© 2017 American Neurological Association.
Conflict of interest statement
Figures


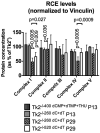

References
-
- Saada A, Shaag A, Mandel H, et al. Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nature genetics. 2001 Nov;29(3):342–4. - PubMed
-
- Lesko N, Naess K, Wibom R, et al. Two novel mutations in thymidine kinase-2 cause early onset fatal encephalomyopathy and severe mtDNA depletion. Neuromuscular disorders : NMD. 2010 Mar;20(3):198–203. - PubMed
-
- Mancuso M, Salviati L, Sacconi S, et al. Mitochondrial DNA depletion: mutations in thymidine kinase gene with myopathy and SMA. Neurology. 2002 Oct 22;59(8):1197–202. - PubMed
-
- Behin A, Jardel C, Claeys KG, et al. Adult cases of mitochondrial DNA depletion due to TK2 defect: an expanding spectrum. Neurology. 2012 Feb 28;78(9):644–8. - PubMed
-
- Tyynismaa H, Sun R, Ahola-Erkkila S, et al. Thymidine kinase 2 mutations in autosomal recessive progressive external ophthalmoplegia with multiple mitochondrial DNA deletions. Human molecular genetics. 2012 Jan 1;21(1):66–75. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

