Short Communication: Comparison of Maxim and Sedia Limiting Antigen Assay Performance for Measuring HIV Incidence
- PMID: 28318310
- PMCID: PMC5467096
- DOI: 10.1089/aid.2016.0245
Short Communication: Comparison of Maxim and Sedia Limiting Antigen Assay Performance for Measuring HIV Incidence
Abstract
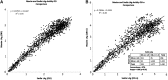
Accurate methods for cross-sectional incidence estimation are needed for HIV prevention research. The Limiting Antigen Avidity (LAg-Avidity) assay has been marketed by two vendors, Maxim Biomedical and Sedia BioSciences Corporation. Performance differences between the two versions of the assay are unknown. We tested a total 1,410 treatment-naive samples with both versions of the assay. The samples came from 176 seroconverters from the Zimbabwe Hormonal Contraception and HIV Study. The correlation between the two versions of the assay was 0.93 for the optical density (OD) and 0.86 for the normalized OD. As the difference was more pronounced for the normalized OD, the difference in assays can be attributed to the calibrators. The mean duration of recent infection (MDRI), the average time individuals infected <2 years appear recently infected, was determined for both versions using an assay cutoff of 1.5 OD-n alone or in combination with a viral load cutoff of >1,000 copies/ml. The MDRI was 137 days for Sedia and 157 days for Maxim, with a difference of 20 days (95% CI 11-30). The MDRIs decreased to 102 and 120 days with the inclusion of a viral load cutoff of >1,000 copies/ml. These results imply that use of the Sedia LAg-Avidity will result in estimates of incidence ∼13% lower than those using the Maxim LAg-Avidity.
Keywords: HIV; cross-sectional incidence testing; subtype C.
Conflict of interest statement
No competing financial interests exist.
Figures
Similar articles
-
Development of an international external quality assurance program for HIV-1 incidence using the Limiting Antigen Avidity assay.PLoS One. 2019 Sep 16;14(9):e0222290. doi: 10.1371/journal.pone.0222290. eCollection 2019. PLoS One. 2019. PMID: 31525218 Free PMC article.
-
Performance comparison of the Maxim and Sedia Limiting Antigen Avidity assays for HIV incidence surveillance.PLoS One. 2019 Jul 26;14(7):e0220345. doi: 10.1371/journal.pone.0220345. eCollection 2019. PLoS One. 2019. PMID: 31348809 Free PMC article.
-
Performance of Bio-Rad and Limiting Antigen Avidity Assays in Detecting Recent HIV Infections Using the Quebec Primary HIV-1 Infection Cohort.PLoS One. 2016 May 25;11(5):e0156023. doi: 10.1371/journal.pone.0156023. eCollection 2016. PLoS One. 2016. PMID: 27224023 Free PMC article. Clinical Trial.
-
Identifying Recent HIV Infections: From Serological Assays to Genomics.Viruses. 2015 Oct 23;7(10):5508-24. doi: 10.3390/v7102887. Viruses. 2015. PMID: 26512688 Free PMC article. Review.
-
Estimates of HIV-1 incidence based on serological methods: a brief methodological review.Cad Saude Publica. 2011;27 Suppl 1:S7-18. doi: 10.1590/s0102-311x2011001300002. Cad Saude Publica. 2011. PMID: 21503527 Review.
Cited by
-
A systematic review of limiting antigen avidity enzyme immunoassay for detection of recent HIV-1 infection to expand supported applications.J Virus Erad. 2022 Sep 7;8(3):100085. doi: 10.1016/j.jve.2022.100085. eCollection 2022 Sep. J Virus Erad. 2022. PMID: 36124229 Free PMC article. Review.
-
Use of HIV Recency Assays for HIV Incidence Estimation and Other Surveillance Use Cases: Systematic Review.JMIR Public Health Surveill. 2022 Mar 11;8(3):e34410. doi: 10.2196/34410. JMIR Public Health Surveill. 2022. PMID: 35275085 Free PMC article.
-
Development of an international external quality assurance program for HIV-1 incidence using the Limiting Antigen Avidity assay.PLoS One. 2019 Sep 16;14(9):e0222290. doi: 10.1371/journal.pone.0222290. eCollection 2019. PLoS One. 2019. PMID: 31525218 Free PMC article.
-
Performance comparison of the Maxim and Sedia Limiting Antigen Avidity assays for HIV incidence surveillance.PLoS One. 2019 Jul 26;14(7):e0220345. doi: 10.1371/journal.pone.0220345. eCollection 2019. PLoS One. 2019. PMID: 31348809 Free PMC article.
References
-
- Murphy G, Parry JV: Assays for the detection of recent infections with human immunodeficiency virus type 1. Euro Surveill 2008;13:pii - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

