Compromised BRCA1-PALB2 interaction is associated with breast cancer risk
- PMID: 28319063
- PMCID: PMC5519427
- DOI: 10.1038/onc.2017.46
Compromised BRCA1-PALB2 interaction is associated with breast cancer risk
Abstract
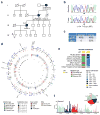
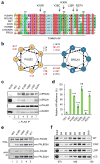
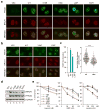
The major breast cancer suppressor proteins BRCA1 and BRCA2 play essential roles in homologous recombination (HR)-mediated DNA repair, which is thought to be critical for tumor suppression. The two BRCA proteins are linked by a third tumor suppressor, PALB2, in the HR pathway. While truncating mutations in these genes are generally pathogenic, interpretation of missense variants remains a challenge. To date, patient-derived missense variants that disrupt PALB2 binding have been identified in BRCA1 and BRCA2; however, there has not been sufficient evidence to prove their pathogenicity in humans, and no variants in PALB2 that disrupt either its BRCA1 or BRCA2 binding have been reported. Here we report on the identification of a novel PALB2 variant, c.104T>C (p.L35P), that segregates in a family with a strong history of breast cancer. Functional analyses showed that L35P abrogates the PALB2-BRCA1 interaction and completely disables its abilities to promote HR and confer resistance to platinum salts and PARP inhibitors. Whole-exome sequencing of a breast cancer from a c.104T>C carrier revealed a second, somatic, truncating mutation affecting PALB2, and the tumor displays hallmark genomic features of tumors with BRCA mutations and HR defects, cementing the pathogenicity of L35P. Parallel analyses of other germline variants in the PALB2 N-terminal BRCA1-binding domain identified multiple variants that affect HR function to varying degrees, suggesting their possible contribution to cancer development. Our findings establish L35P as the first pathogenic missense mutation in PALB2 and directly demonstrate the requirement of the PALB2-BRCA1 interaction for breast cancer suppression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Xia B, Sheng Q, Nakanishi K, Ohashi A, Wu J, Christ N, et al. Control of BRCA2 cellular and clinical functions by a nuclear partner, PALB2. Mol Cell. 2006;22:719–729. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

