Iron addiction: a novel therapeutic target in ovarian cancer
- PMID: 28319068
- PMCID: PMC5540148
- DOI: 10.1038/onc.2017.11
Iron addiction: a novel therapeutic target in ovarian cancer
Abstract
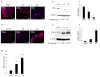
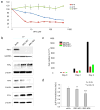
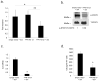
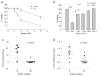
Ovarian cancer is a lethal malignancy that has not seen a major therapeutic advance in over 30 years. We demonstrate that ovarian cancer exhibits a targetable alteration in iron metabolism. Ferroportin (FPN), the iron efflux pump, is decreased, and transferrin receptor (TFR1), the iron importer, is increased in tumor tissue from patients with high grade but not low grade serous ovarian cancer. A similar profile of decreased FPN and increased TFR1 is observed in a genetic model of ovarian cancer tumor-initiating cells (TICs). The net result of these changes is an accumulation of excess intracellular iron and an augmented dependence on iron for proliferation. A forced reduction in intracellular iron reduces the proliferation of ovarian cancer TICs in vitro, and inhibits both tumor growth and intraperitoneal dissemination of tumor cells in vivo. Mechanistic studies demonstrate that iron increases metastatic spread by facilitating invasion through expression of matrix metalloproteases and synthesis of interleukin 6 (IL-6). We show that the iron dependence of ovarian cancer TICs renders them exquisitely sensitive in vivo to agents that induce iron-dependent cell death (ferroptosis) as well as iron chelators, and thus creates a metabolic vulnerability that can be exploited therapeutically.
Conflict of interest statement
Figures




References
-
- A snapshot of ovarian cancer. [cited 2015 December 21 2015]. Available from: http://www.cancer.gov/research/progress/snapshots/ovarian.
-
- Leong HS, Galletta L, Etemadmoghadam D, George J, Kobel M, Ramus SJ, et al. Efficient molecular subtype classification of high-grade serous ovarian cancer. J Pathol. 2015 Jul;236(3):272–7. Epub 2015/03/27. eng. - PubMed
-
- Tothill RW, Tinker AV, George J, Brown R, Fox SB, Lade S, et al. Novel molecular subtypes of serous and endometrioid ovarian cancer linked to clinical outcome. Clin Cancer Res. 2008 Aug 15;14(16):5198–208. Epub 2008/08/14. eng. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

