Late Effects of Exposure to Ionizing Radiation and Age on Human Thymus Morphology and Function
- PMID: 28319462
- PMCID: PMC5505072
- DOI: 10.1667/RR4554.1
Late Effects of Exposure to Ionizing Radiation and Age on Human Thymus Morphology and Function
Abstract
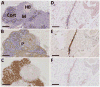
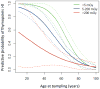
The thymus is essential for proper development and maintenance of a T-cell repertoire that can respond to newly encountered antigens, but its function can be adversely affected by internal factors such as pregnancy and normal aging or by external stimuli such as stress, infection, chemotherapy and ionizing radiation. We have utilized a unique archive of thymus tissues, obtained from 165 individuals, exposed to the 1945 atomic bomb blast in Hiroshima, to study the long-term effects of receiving up to ∼3 Gy dose of ionizing radiation on human thymus function. A detailed morphometric analysis of thymus activity and architecture in these subjects at the time of their natural deaths was performed using bright-field immunohistochemistry and dual-color immunofluorescence and compared to a separate cohort of nonexposed control subjects. After adjusting for age-related effects, increased hallmarks of thymic involution were observed histologically in individuals exposed to either low (5-200 mGy) or moderate-to-high (>200 mGy) doses of ionizing radiation compared to unirradiated individuals (<5 mGy). Sex-related differences were seen when the analysis was restricted to individuals under 60 years of attained age at sample collection, but were not observed when comparing across the entire age range. This indicates that while females undergo slower involution than males, they ultimately attain similar phenotypes. These findings suggest that even low-dose-radiation exposure can accelerate thymic aging, with decreased thymopoiesis relative to nonexposed controls evident years after exposure. These data were used to develop a model that can predict thymic function during normal aging or in individuals therapeutically or accidentally exposed to radiation.
Figures

References
-
- Mackall CL, Punt JA, Morgan P, Farr AG, Gress RE. Thymic function in young/old chimeras: substantial thymic T cell regenerative capacity despite irreversible age-associated thymic involution. Eur J Immunol. 1998;28:1886–93. - PubMed
-
- Zhu X, Gui J, Dohkan J, Cheng L, Barnes PF, Su DM. Lymphohematopoietic progenitors do not have a synchronized defect with age-related thymic involution. Aging Cell. 2007;6:663–72. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

