Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow
- PMID: 28319610
- PMCID: PMC5404963
- DOI: 10.1038/nn.4533
Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow
Abstract
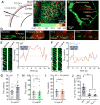
Blood flow into the brain is dynamically regulated to satisfy the changing metabolic requirements of neurons, but how this is accomplished has remained unclear. Here we demonstrate a central role for capillary endothelial cells in sensing neural activity and communicating it to upstream arterioles in the form of an electrical vasodilatory signal. We further demonstrate that this signal is initiated by extracellular K+ -a byproduct of neural activity-which activates capillary endothelial cell inward-rectifier K+ (KIR2.1) channels to produce a rapidly propagating retrograde hyperpolarization that causes upstream arteriolar dilation, increasing blood flow into the capillary bed. Our results establish brain capillaries as an active sensory web that converts changes in external K+ into rapid, 'inside-out' electrical signaling to direct blood flow to active brain regions.
Conflict of interest statement
None.
Figures



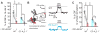
Comment in
-
Upstream current for a downstream flow.Nat Neurosci. 2017 Apr 25;20(5):631-633. doi: 10.1038/nn.4542. Nat Neurosci. 2017. PMID: 28440803 No abstract available.
References
-
- Iadecola C, Nedergaard M. Glial regulation of the cerebral microvasculature. Nat Neurosci. 2007;10:1369–1376. - PubMed
MeSH terms
Substances
Grants and funding
- R01 HL121706/HL/NHLBI NIH HHS/United States
- K01 DK103840/DK/NIDDK NIH HHS/United States
- T32 HL007594/HL/NHLBI NIH HHS/United States
- UM1 HL120877/HL/NHLBI NIH HHS/United States
- R37 DK053832/DK/NIDDK NIH HHS/United States
- R01 HL131181/HL/NHLBI NIH HHS/United States
- R01 HL136636/HL/NHLBI NIH HHS/United States
- S10 OD010583/OD/NIH HHS/United States
- 14POST20480144/AHA/American Heart Association-American Stroke Association/United States
- P01 HL095488/HL/NHLBI NIH HHS/United States
- P30 GM103498/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

