31P-dephased, 13C-detected REDOR for NMR crystallography at natural isotopic abundance
- PMID: 28319851
- PMCID: PMC5478420
- DOI: 10.1016/j.jmr.2017.02.020
31P-dephased, 13C-detected REDOR for NMR crystallography at natural isotopic abundance
Abstract
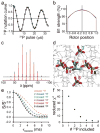
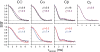
Typically, the process of NMR-based structure determination relies on accurately measuring a large number of internuclear distances to serve as restraints for simulated annealing calculations. In solids, the rotational-echo double-resonance (REDOR) experiment is a widely used approach to determine heteronuclear dipolar couplings corresponding to distances usually in the range of 1.5-8Å. A challenge in the interpretation of REDOR data is the degeneracy of symmetric subunits in an oligomer or equivalent molecules in a crystal lattice, which produce REDOR trajectories that depend explicitly on two or more distances instead of one. This degeneracy cannot be overcome by either spin dilution (for molecules containing 31P, 19F and other highly abundant nuclei) or selective pulses (in the case where there is chemical shift degeneracy). For small, crystalline molecules, such as phosphoserine, we demonstrate that as many as five inter-molecular distances must be considered to model 31P-dephased REDOR data accurately. We report excellent agreement between simulation and experiment once lattice couplings, 31P chemical shift anisotropy, and radio-frequency field inhomogeneity are all taken into account. We also discuss the systematic inaccuracies that may result from approximations that consider only the initial slope of the REDOR trajectory and/or that utilize a two- or three-spin system. Furthermore, we demonstrate the applicability of 31P-dephased REDOR for validation or refinement of candidate crystal structures and show that this approach is especially informative for NMR crystallography of 31P-containing molecules.
Keywords: Magic-angle spinning; Phosphorus; Phosphoserine; Simulation; Solid-state NMR.
Copyright © 2017 Elsevier Inc. All rights reserved.
Figures




References
-
- Sun HH, Sanders LK, Oldfield E. Carbon-13 NMR shielding in the twenty common amino acids: Comparisons with experimental results in proteins. J Am Chem Soc. 2002;124:5486–5495. - PubMed
-
- Salager E, Day GM, Stein RS, Pickard CJ, Elena B, Emsley L. Powder Crystallography by Combined Crystal Structure Prediction and High-Resolution H-1 Solid-State NMR Spectroscopy. J Am Chem Soc. 2010;132:2564–+. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

