Towards a Unified Testing Framework for Single-Sided Deafness Studies: A Consensus Paper
- PMID: 28319951
- PMCID: PMC5472212
- DOI: 10.1159/000455058
Towards a Unified Testing Framework for Single-Sided Deafness Studies: A Consensus Paper
Abstract
Background: While hearing aids for a contralateral routing of signals (CROS-HA) and bone conduction devices have been the traditional treatment for single-sided deafness (SSD) and asymmetric hearing loss (AHL), in recent years, cochlear implants (CIs) have increasingly become a viable treatment choice, particularly in countries where regulatory approval and reimbursement schemes are in place. Part of the reason for this shift is that the CI is the only device capable of restoring bilateral input to the auditory system and hence of possibly reinstating binaural hearing. Although several studies have independently shown that the CI is a safe and effective treatment for SSD and AHL, clinical outcome measures in those studies and across CI centers vary greatly. Only with a consistent use of defined and agreed-upon outcome measures across centers can high-level evidence be generated to assess the safety and efficacy of CIs and alternative treatments in recipients with SSD and AHL.
Methods: This paper presents a comparative study design and minimum outcome measures for the assessment of current treatment options in patients with SSD/AHL. The protocol was developed, discussed, and eventually agreed upon by expert panels that convened at the 2015 APSCI conference in Beijing, China, and at the CI 2016 conference in Toronto, Canada.
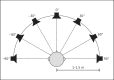
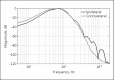
Results: A longitudinal study design comparing CROS-HA, BCD, and CI treatments is proposed. The recommended outcome measures include (1) speech in noise testing, using the same set of 3 spatial configurations to compare binaural benefits such as summation, squelch, and head shadow across devices; (2) localization testing, using stimuli that rove in both level and spectral content; (3) questionnaires to collect quality of life measures and the frequency of device use; and (4) questionnaires for assessing the impact of tinnitus before and after treatment, if applicable.
Conclusion: A protocol for the assessment of treatment options and outcomes in recipients with SSD and AHL is presented. The proposed set of minimum outcome measures aims at harmonizing assessment methods across centers and thus at generating a growing body of high-level evidence for those treatment options.
Keywords: Asymmetric hearing loss; Bimodal stimulation; Bone conduction device; Bone-anchored hearing aid; Cochlear implant; Contralateral routing of signals hearing aid; Single-sided deafness; Testing method consensus; Unilateral hearing loss.
© 2017 The Author(s) Published by S. Karger AG, Basel.
Figures
References
-
- Arndt S, Aschendorff A, Laszig R, Beck R, Schild C, Kroeger S, Ihorst G, Wesarg T. Comparison of pseudobinaural hearing to real binaural hearing rehabilitation after cochlear implantation in patients with unilateral deafness and tinnitus. Otol Neurotol. 2011;32:39–47. - PubMed
-
- Arndt S, Laszig R, Aschendorff A, Hassepass F, Beck R, Wesarg T. Cochlear implant treatment of patients with single-sided deafness or asymmetric hearing loss. HNO. 2017 Epub ahead of print. - PubMed
-
- Bronkhorst AW, Plomp R. The effect of head-induced interaural time and level differences on speech intelligibility in noise. J Acoust Soc Am. 1988;83:1508–1516. - PubMed
-
- Bruce I, Cooper H, Waltzman S, Schramm D, Graham J. Editorial maximising research value in the field of hearing implantation: a call for “big data”. Cochlear Implants Int. 2015;16:301–302. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical