Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review
- PMID: 28319996
- PMCID: PMC5486987
- DOI: 10.7326/M16-2575
Oral Direct-Acting Agent Therapy for Hepatitis C Virus Infection: A Systematic Review
Abstract
Background: Rapid improvements in hepatitis C virus (HCV) therapy have led to the approval of multiple oral direct-acting antiviral (DAA) regimens by the U.S. Food and Drug Administration (FDA) for treatment of chronic HCV infection.
Purpose: To summarize published literature on the efficacy and safety of oral DAAs for treatment of persons with chronic HCV infection.
Data sources: MEDLINE and EMBASE from inception through 1 November 2016.
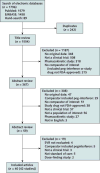
Study selection: 42 English-language studies from controlled and single-group registered clinical trials of adults with HCV infection that evaluated at least 8 weeks of an FDA-approved interferon-free HCV regimen that included at least 2 DAAs.
Data extraction: Two investigators abstracted data on study design, patient characteristics, and virologic and safety outcomes sequentially and assessed quality independently.
Data synthesis: Six DAA regimens showed high sustained virologic response (SVR) rates (>95%) in patients with HCV genotype 1 infection without cirrhosis, including those with HIV co-infection. Effective treatments for HCV genotype 3 infection are limited (2 DAA regimens). Patients with hepatic decompensation, particularly those with Child-Turcotte-Pugh class C disease, had lower SVR rates (78% to 87%) than other populations. The addition of ribavirin was associated with increased SVR rates for certain DAA regimens and patient groups. Overall rates of serious adverse events and treatment discontinuation were low (<10% in the general population); regimens that included ribavirin had more mild or moderate adverse events than those without.
Limitations: Twenty-three studies had moderate risk of bias (10 were open-label single-group trials, 11 had limited information on concealment of the allocation scheme, and 5 had selective outcome reporting). All but 1 of the studies were industry-funded. Heterogeneity of interventions precluded pooling.
Conclusion: Multiple oral DAA regimens show high rates of safety, tolerability, and efficacy for treatment of HCV genotype 1 infection, particularly among persons without cirrhosis.
Primary funding source: Patient-Centered Outcomes Research Institute. (PROSPERO: CRD42014009711).
Conflict of interest statement
Authors not named here have disclosed no conflicts of interest.
Figures


Comment in
-
Hepatitis C: Down but Not Out.Ann Intern Med. 2017 May 2;166(9):675-676. doi: 10.7326/M17-0567. Epub 2017 Mar 21. Ann Intern Med. 2017. PMID: 28319998 No abstract available.
-
Review: In chronic hepatitis C virus infection, oral direct-acting antivirals have high sustained virologic response.Ann Intern Med. 2017 Jul 18;167(2):JC3. doi: 10.7326/ACPJC-2017-167-2-003. Ann Intern Med. 2017. PMID: 28715825 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
