Constitutive androstane receptor regulates the intestinal mucosal response to injury
- PMID: 28320072
- PMCID: PMC5446585
- DOI: 10.1111/bph.13787
Constitutive androstane receptor regulates the intestinal mucosal response to injury
Abstract
Background and purpose: The pathogenesis of the inflammatory bowel diseases (IBD), comprising Crohn's disease (CD) and ulcerative colitis (UC), involves aberrant interactions between a genetically susceptible individual, their microbiota and environmental factors. Alterations in xenobiotic receptor expression and function are associated with increased risk for IBD. Here, we have assessed the role of the constitutive androstane receptor (CAR), a xenobiotic receptor closely related to the pregnane X receptor, in the regulation of intestinal mucosal homeostasis.
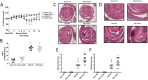
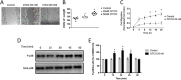
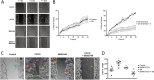
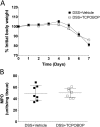
Experimental approach: CAR expression was assessed in intestinal mucosal biopsies obtained from CD and UC patients, and in C57/Bl6 mice exposed to dextran sulphate sodium (DSS; 3.5% w/v in drinking water) to evoke intestinal inflammation and tissue damage. CAR-deficient mice were exposed to DSS and mucosal healing assessed. Modulation of wound healing by CAR was assessed in vitro. The therapeutic potential of CAR activation was evaluated, using 3,3',5,5'-tetrachloro-1,4-bis(pyridyloxy)benzene (TCPOBOP), a selective rodent CAR agonist.
Key results: CAR expression was reduced in CD and UC samples, compared with expression in healthy controls. This was reproduced in our DSS studies, where CAR expression was reduced in colitic mice. CAR-deficient mice exhibited reduced healing following DSS exposure. In vitro, CAR activation accelerated intestinal epithelial wound healing by enhancing cell migration. Lastly, treating mice with TCPOBOP, following induction of colitis, enhanced mucosal healing.
Conclusion and implications: Our results support the notion that xenobiotic sensing is altered during intestinal inflammation, and suggest that CAR activation may prove effective in enhancing mucosal healing in patients with IBD.
© 2017 The British Pharmacological Society.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

