Targets for adapting intravenous iron dose in hemodialysis: a proof of concept study
- PMID: 28320343
- PMCID: PMC5358046
- DOI: 10.1186/s12882-017-0513-x
Targets for adapting intravenous iron dose in hemodialysis: a proof of concept study
Abstract
Background: Intravenous iron is widely used to control anemia in dialysis patients and limits costs related to erythropoiesis-stimulating agents (ESA). Current guidelines do not clearly set upper limits for serum ferritin (SF) and transferrin saturation (TSAT). International surveys such as the Dialysis Outcomes and Practice Patterns Study (DOPPS) showed that this lack of upper limits potentially led nephrologists to prescribe iron infusions even for patients with a high SF. Recent publications have suggested a risk of short- and long-term adverse effects related to iron overload. We conducted a proof of concept study to assess the impact of reducing intravenous iron administration.
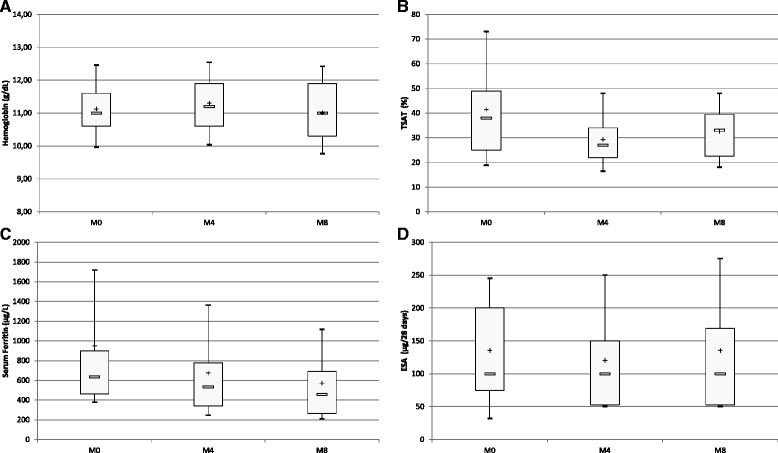
Methods: In a prospective 8-month study conducted in a hospital dialysis unit, we assessed the impact of a strategy designed to reduce iron infusions. Instead of the usual strategy targeting 30-50% TSAT irrespective of SF, intravenous iron was administered if and only if TSAT was below 20% and SF below 200 μg/L. Routine practices for ESA remained unchanged: hemoglobin target 10-12 g/dL; ESA delivered monthly and dose corrected by 25% as necessary; ESA discontinued temporarily if hemoglobin >13 g/dL; methoxy polyethylene glycol-epoetin beta generally used. Tests were ordered monthly to monitor hemoglobin. Intravenous iron was administered weekly and ESA monthly. Baseline and 6-month TSAT, SF and hemoglobin levels were compared.
Results: Six-month data were available for 45 patients (31 M/14 F; 67.6 ± 14.0 y; 53.9 ± 85.7 months on dialysis). Patients experienced the following comorbidities: ischemic heart disease (n = 29, 44%), diabetes mellitus (n = 14; 31%), malignant disease (n = 11; 24%), transplantation (n = 11; 24%) and severe heart failure (n = 6; 13%). The mean weekly dose of iron declined from 77.8 ± 87.6 to 24.4 ± 52.9 mg per patient (p = 0.0003). SF decreased from 947.7 ± 1056.4 to 570.7 ± 424.4 μg/L (p = 0.0001), and TSAT from 41.5 ± 22.4 to 32.6 ± 13.7% (p = 0.01). Hemoglobin levels remained stable (11.13 ± 1.05 vs. 11.00 ± 1.16 g/dL, p = 0.54) as did ESA dose (126.4 ± 91.9 vs. 108.2 ± 112.7 μg/28 days, p = 0.07).
Conclusions: Our study suggests that a regular hemoglobin level can be maintained using regular ESA doses combined with intravenous iron doses adapted to TSAT and SF thresholds lower than those used in routine practice. This strategy reduces the risk of iron overload.
Keywords: Anemia; Chronic kidney disease; Dialysis; Intravenous iron.
Figures

References
-
- Collins AJ, Li S, St Peter W, Ebben J, Roberts T, Ma JZ, Manning W. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39% J Am Soc Nephrol. 2001;12:2465–73. - PubMed
-
- Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–90. doi: 10.1056/NEJM199808273390903. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

