Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health ("NiPPeR"): study protocol for a randomised controlled trial
- PMID: 28320484
- PMCID: PMC5359891
- DOI: 10.1186/s13063-017-1875-x
Nutritional Intervention Preconception and During Pregnancy to Maintain Healthy Glucose Metabolism and Offspring Health ("NiPPeR"): study protocol for a randomised controlled trial
Abstract
Background: Improved maternal nutrition and glycaemic control before and during pregnancy are thought to benefit the health of the mother, with consequent benefits for infant body composition and later obesity risk. Maternal insulin resistance and glycaemia around conception and in early pregnancy may be key determinants of maternal physiology and placental function, affecting fetal nutrient supply and maternal-feto-placental communications throughout gestation, with implications for later postnatal health.
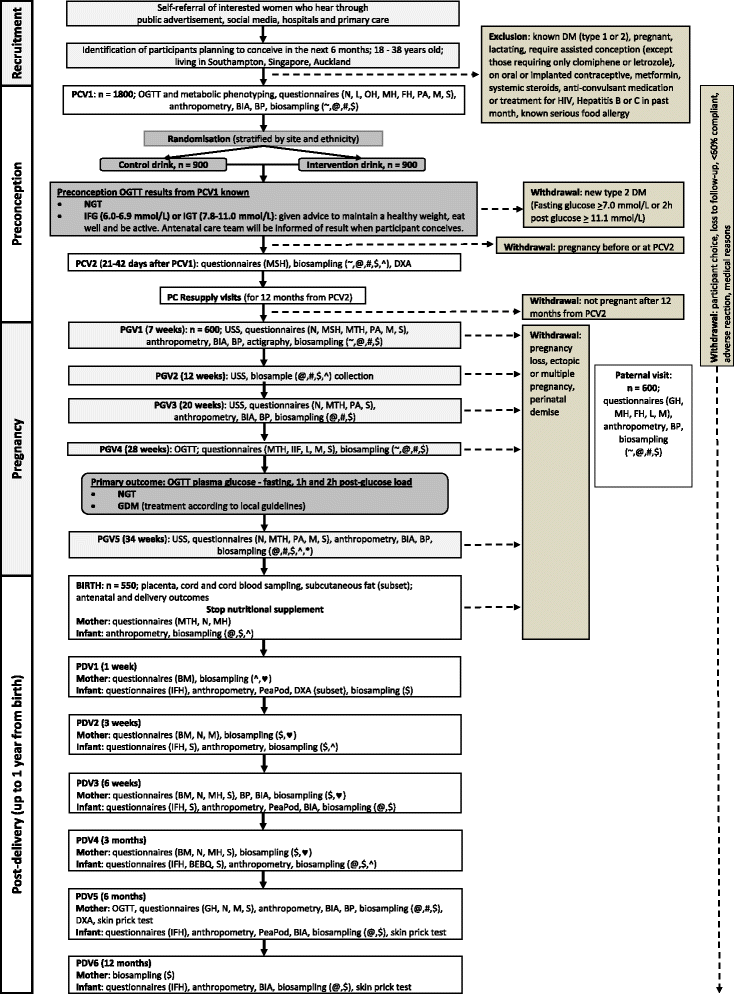
Methods/design: This double-blind randomised controlled trial will recruit up to 1800 women, aged 18-38 years, who are planning a pregnancy in the United Kingdom (UK), Singapore and New Zealand, with a view to studying 600 pregnancies. The primary outcome is maternal glucose tolerance at 28 weeks' gestation following an oral glucose tolerance test. Secondary outcomes include metabolic, molecular and health-related outcomes in the mother and offspring, notably infant body composition. Participants will be randomly allocated to receive a twice-daily control nutritional drink, enriched with standard micronutrients, or a twice-daily intervention nutritional drink enriched with additional micronutrients, myo-inositol and probiotics, both demonstrated previously to assist in maintaining healthy glucose metabolism during pregnancy. Myo-inositol is a nutrient that enhances cellular glucose uptake. The additional micronutrients seek to address deficiencies of some B-group vitamins and vitamin D that are both common during pregnancy and that have been associated with maternal dysglycaemia, epigenetic changes and greater offspring adiposity. Women who conceive within a year of starting the nutritional drinks will be followed through pregnancy and studied with their infants at six time points during the first year of life. Blood, urine/stool, hair and cheek swabs will be collected from the mothers for genetic, epigenetic, hormone, nutrient and metabolite measurements, and assessments of the mother's body composition, anthropometry, health, diet and lifestyle will be made. Infants will also undergo hair, cheek swab, urine and stool sampling for similar biological measurements; infant body composition will be assessed and feeding recorded.
Discussion: There is an increasing focus on the need to optimise maternal nutrition starting prior to conception. This trial will provide evidence on the potential for nutritional interventions beginning prior to conception to promote healthy maternal and offspring outcomes.
Trial registration: ClinicalTrials.gov, identifier: NCT02509988 , Universal Trial Number U1111-1171-8056. Registered on 16 July 2015. This is an academic-led study by the EpiGen Global Research Consortium.
Keywords: Body composition; Glucose metabolism; Hyperglycemia; Metabolic diseases; Nutrition; Preconception; Pregnancy; Randomised trial.
Figures
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- PG/14/33/30827/BHF_/British Heart Foundation/United Kingdom
- RG/15/17/31749/BHF_/British Heart Foundation/United Kingdom
- MC_U147585827/MRC_/Medical Research Council/United Kingdom
- 21231/ARC_/Arthritis Research UK/United Kingdom
- 17702/ARC_/Arthritis Research UK/United Kingdom
- MC_UP_A620_1014/MRC_/Medical Research Council/United Kingdom
- MC_UP_A620_1017/MRC_/Medical Research Council/United Kingdom
- MC_U147574222/MRC_/Medical Research Council/United Kingdom
- HTA/10/33/04/DH_/Department of Health/United Kingdom
- MC_UU_12011/4/MRC_/Medical Research Council/United Kingdom
- G0400491/MRC_/Medical Research Council/United Kingdom
- MC_U147585824/MRC_/Medical Research Council/United Kingdom
- MC_U147585819/MRC_/Medical Research Council/United Kingdom
- MC_U147574226/MRC_/Medical Research Council/United Kingdom
- 10/33/04/DH_/Department of Health/United Kingdom
- MC_UU_12011/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

