Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1
- PMID: 28320709
- PMCID: PMC5445571
- DOI: 10.1182/blood-2016-09-742825
Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1
Erratum in
-
Sugimoto MA, Ribeiro ALC, Costa BRC, et al. Plasmin and plasminogen induce macrophage reprogramming and regulate key steps of inflammation resolution via annexin A1. Blood. 2017;129(21):2896-2907.Blood. 2018 Jul 26;132(4):459. doi: 10.1182/blood-2018-05-854158. Blood. 2018. PMID: 30049736 Free PMC article. No abstract available.
Abstract
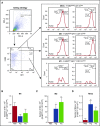
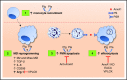
Inflammation resolution is an active process that functions to restore tissue homeostasis. The participation of the plasminogen (Plg)/plasmin (Pla) system in the productive phase of inflammation is well known, but its involvement in the resolution phase remains unclear. Therefore, we aimed to investigate the potential role of Plg/Pla in key events during the resolution of acute inflammation and its underlying mechanisms. Plg/Pla injection into the pleural cavity of BALB/c mice induced a time-dependent influx of mononuclear cells that were primarily macrophages of anti-inflammatory (M2 [F4/80high Gr1- CD11bhigh]) and proresolving (Mres [F4/80med CD11blow]) phenotypes, without changing the number of macrophages with a proinflammatory profile (M1 [F4/80low Gr1+ CD11bmed]). Pleural injection of Plg/Pla also increased M2 markers (CD206 and arginase-1) and secretory products (transforming growth factor β and interleukin-6) and decreased the expression of inducible nitric oxide synthase (M1 marker). During the resolving phase of lipopolysaccharide (LPS)-induced inflammation when resolving macrophages predominate, we found increased Plg expression and Pla activity, further supporting a link between the Plg/Pla system and key cellular events in resolution. Indeed, Plg or Pla given at the peak of inflammation promoted resolution by decreasing neutrophil numbers and increasing neutrophil apoptosis and efferocytosis in a serine-protease inhibitor-sensitive manner. Next, we confirmed the ability of Plg/Pla to both promote efferocytosis and override the prosurvival effect of LPS via annexin A1. These findings suggest that Plg and Pla regulate several key steps in inflammation resolution, namely, neutrophil apoptosis, macrophage reprogramming, and efferocytosis, which have a major impact on the establishment of an efficient resolution process.
© 2017 by The American Society of Hematology.
Figures





Comment in
-
Reprogramming macrophages by plasmin.Blood. 2017 May 25;129(21):2823-2824. doi: 10.1182/blood-2017-04-776377. Blood. 2017. PMID: 28546223 No abstract available.
References
-
- Sousa LP, Alessandri AL, Pinho V, Teixeira MM. Pharmacological strategies to resolve acute inflammation. Curr Opin Pharmacol. 2013;13(4):625-631. - PubMed
-
- Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140(6):871-882. - PubMed
-
- Alessandri AL, Sousa LP, Lucas CD, Rossi AG, Pinho V, Teixeira MM. Resolution of inflammation: mechanisms and opportunity for drug development. Pharmacol Ther. 2013;139(2):189-212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

