In Vitro Activity of Imipenem-Relebactam against Gram-Negative ESKAPE Pathogens Isolated by Clinical Laboratories in the United States in 2015 (Results from the SMART Global Surveillance Program)
- PMID: 28320716
- PMCID: PMC5444184
- DOI: 10.1128/AAC.02209-16
In Vitro Activity of Imipenem-Relebactam against Gram-Negative ESKAPE Pathogens Isolated by Clinical Laboratories in the United States in 2015 (Results from the SMART Global Surveillance Program)
Abstract
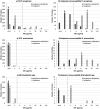
Relebactam (formerly MK-7655) is an inhibitor of class A and C β-lactamases, including Klebsiella pneumoniae carbapenemase (KPC), and is currently in clinical development in combination with imipenem-cilastatin. Using Clinical and Laboratory Standards Institute (CLSI)-defined broth microdilution methodology, we evaluated the in vitro activities of imipenem-relebactam, imipenem, and seven routinely tested parenteral antimicrobial agents against Gram-negative ESKAPE pathogens (including Klebsiella pneumoniae, n = 689; Acinetobacter baumannii, n = 72; Pseudomonas aeruginosa, n = 845; and Enterobacter spp., n = 399) submitted by 21 clinical laboratories in the United States in 2015 as part of the SMART (Study for Monitoring Antimicrobial Resistance Trends) global surveillance program. Relebactam was tested at a fixed concentration of 4 μg/ml in combination with doubling dilutions of imipenem. Imipenem-relebactam MICs were interpreted using CLSI imipenem breakpoints. The respective rates of susceptibility to imipenem-relebactam and imipenem were 94.2% (796/845) and 70.3% (594/845) for P. aeruginosa, 99.0% (682/689) and 96.1% (662/689) for K. pneumoniae, and 100% (399/399) and 98.0% (391/399) for Enterobacter spp. Relebactam restored imipenem susceptibility to 80.5% (202/251), 74.1% (20/27), and 100% (8/8) of isolates of imipenem-nonsusceptible P. aeruginosa, K. pneumoniae, and Enterobacter spp. Relebactam did not increase the number of isolates of Acinetobacter spp. susceptible to imipenem, and the rates of resistance to all of the agents tested against this pathogen were >30%. Further development of imipenem-relebactam is warranted given the demonstrated ability of relebactam to restore the activity of imipenem against current clinical isolates of Enterobacteriaceae and P. aeruginosa that are nonsusceptible to carbapenems and its potential as a therapy for treating patients with antimicrobial-resistant Gram-negative infections.
Keywords: ESKAPE pathogens; SMART; imipenem; relebactam; surveillance.
Copyright © 2017 American Society for Microbiology.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

