Ciliary Mechanisms of Cyst Formation in Polycystic Kidney Disease
- PMID: 28320755
- PMCID: PMC5666631
- DOI: 10.1101/cshperspect.a028209
Ciliary Mechanisms of Cyst Formation in Polycystic Kidney Disease
Abstract
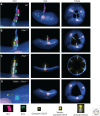
Autosomal-dominant polycystic kidney disease (ADPKD) is a disease of defective tissue homeostasis resulting in active remodeling of nephrons and bile ducts to form fluid-filled sacs called cysts. The causal genes PKD1 and PKD2 encode transmembrane proteins polycystin 1 (PC1) and polycystin 2 (PC2), respectively. Together, the polycystins localize to the solitary primary cilium that protrudes from the apical surface of most kidney tubule cells and is thought to function as a privileged compartment that the cell uses for signal integration of sensory inputs. It has been proposed that PC1 and PC2 form a receptor-channel complex that detects external stimuli and transmit a local calcium-mediated signal, which may control a multitude of cellular processes by an as-yet unknown mechanism. Genetic studies using mouse models of cilia and polycystin dysfunction have shown that polycystins regulate an unknown cilia-dependent signal that is normally part of the homeostatic maintenance of nephron structure. ADPKD ensues when this pathway is dysregulated by absence of polycystins from intact cilia, but disruption of cilia also disrupts this signaling mechanism and ameliorates ADPKD even in the absence of polycystins. Understanding the role of cilia and ciliary signaling in ADPKD is challenging, but success will provide saltatory advances in our understanding of how tubule structure is maintained in healthy kidneys and how disruption of polycystin or cilia function leads to the pathological tissue remodeling process underlying ADPKD.
Copyright © 2017 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
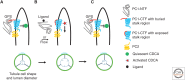
References
-
- Audrezet MP, Cornec-Le Gall E, Chen JM, Redon S, Quere I, Creff J, Benech C, Maestri S, Le Meur Y, Ferec C. 2012. Autosomal dominant polycystic kidney disease: Comprehensive mutation analysis of PKD1 and PKD2 in 700 unrelated patients. Hum Mutat 33: 1239–1250. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
