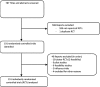
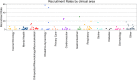
Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme
- PMID: 28320800
- PMCID: PMC5372123
- DOI: 10.1136/bmjopen-2016-015276
Recruitment and retention of participants in randomised controlled trials: a review of trials funded and published by the United Kingdom Health Technology Assessment Programme
Abstract
Background: Substantial amounts of public funds are invested in health research worldwide. Publicly funded randomised controlled trials (RCTs) often recruit participants at a slower than anticipated rate. Many trials fail to reach their planned sample size within the envisaged trial timescale and trial funding envelope.
Objectives: To review the consent, recruitment and retention rates for single and multicentre randomised control trials funded and published by the UK's National Institute for Health Research (NIHR) Health Technology Assessment (HTA) Programme.
Data sources and study selection: HTA reports of individually randomised single or multicentre RCTs published from the start of 2004 to the end of April 2016 were reviewed.
Data extraction: Information was extracted, relating to the trial characteristics, sample size, recruitment and retention by two independent reviewers.
Main outcome measures: Target sample size and whether it was achieved; recruitment rates (number of participants recruited per centre per month) and retention rates (randomised participants retained and assessed with valid primary outcome data).
Results: This review identified 151 individually RCTs from 787 NIHR HTA reports. The final recruitment target sample size was achieved in 56% (85/151) of the RCTs and more than 80% of the final target sample size was achieved for 79% of the RCTs (119/151). The median recruitment rate (participants per centre per month) was found to be 0.92 (IQR 0.43-2.79) and the median retention rate (proportion of participants with valid primary outcome data at follow-up) was estimated at 89% (IQR 79-97%).
Conclusions: There is considerable variation in the consent, recruitment and retention rates in publicly funded RCTs. Investigators should bear this in mind at the planning stage of their study and not be overly optimistic about their recruitment projections.
Keywords: Health Technology Assessment; Publicly funded; Randomised Controlled Trials; Retention; recruitment; review.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Figures
References
-
- National Institute for Health Research Annual Report 2014/15 (online). http://www.nihr.ac.uk/news/nihr-annual-report-201415/3111 (accessed 28 Feb 2017).
-
- Prescott RJ, Counsell CE, Gillespie WJ et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess (Winchester, England). 1999;3:1–143. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous