STAT3 Controls the Long-Term Survival and Phenotype of Repair Schwann Cells during Nerve Regeneration
- PMID: 28320842
- PMCID: PMC5413174
- DOI: 10.1523/JNEUROSCI.3481-16.2017
STAT3 Controls the Long-Term Survival and Phenotype of Repair Schwann Cells during Nerve Regeneration
Abstract
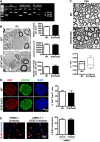
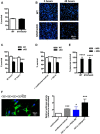
After nerve injury, Schwann cells convert to a phenotype specialized to promote repair. But during the slow process of axonal regrowth, these repair Schwann cells gradually lose their regeneration-supportive features and eventually die. Although this is a key reason for the frequent regeneration failures in humans, the transcriptional mechanisms that control long-term survival and phenotype of repair cells have not been studied, and the molecular signaling underlying their decline is obscure. We show, in mice, that Schwann cell STAT3 has a dual role. It supports the long-term survival of repair Schwann cells and is required for the maintenance of repair Schwann cell properties. In contrast, STAT3 is less important for the initial generation of repair Schwann cells after injury. In repair Schwann cells, we find that Schwann cell STAT3 activation by Tyr705 phosphorylation is sustained during long-term denervation. STAT3 is required for maintaining autocrine Schwann cell survival signaling, and inactivation of Schwann cell STAT3 results in a striking loss of repair cells from chronically denervated distal stumps. STAT3 inactivation also results in abnormal morphology of repair cells and regeneration tracks, and failure to sustain expression of repair cell markers, including Shh, GDNF, and BDNF. Because Schwann cell development proceeds normally without STAT3, the function of this factor appears restricted to Schwann cells after injury. This identification of transcriptional mechanisms that support long-term survival and differentiation of repair cells will help identify, and eventually correct, the failures that lead to the deterioration of this important cell population.SIGNIFICANCE STATEMENT Although injured peripheral nerves contain repair Schwann cells that provide signals and spatial clues for promoting regeneration, the clinical outcome after nerve damage is frequently poor. A key reason for this is that, during the slow growth of axons through the proximal parts of injured nerves repair, Schwann cells gradually lose regeneration-supporting features and eventually die. Identification of signals that sustain repair cells is therefore an important goal. We have found that in mice the transcription factor STAT3 protects these cells from death and contributes to maintaining the molecular and morphological repair phenotype that promotes axonal regeneration. Defining the molecular mechanisms that maintain repair Schwann cells is an essential step toward developing therapeutic strategies that improve nerve regeneration and functional recovery.
Keywords: Schwann; denervation; injury; nerve; regeneration; repair.
Copyright © 2017 Benito, Davis et al.
Figures






References
-
- Allan CH. (2000) Functional results of primary nerve repair. Hand Clin 16:67–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
