A biophysical vascular bubble model for devising decompression procedures
- PMID: 28320890
- PMCID: PMC5371562
- DOI: 10.14814/phy2.13191
A biophysical vascular bubble model for devising decompression procedures
Abstract
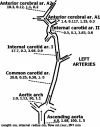
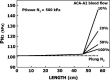
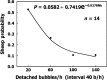
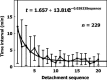
Vascular bubble models, which present a realistic biophysical approach, hold great promise for devising suitable diver decompression procedures. Nanobubbles were found to nucleate on a flat hydrophobic surface, expanding to form bubbles after decompression. Such active hydrophobic spots (AHS) were formed from lung surfactants on the luminal aspect of ovine blood vessels. Many of the phenomena observed in these bubbling vessels correlated with those known to occur in diving. On the basis of our previous studies, which proposed a new model for the formation of arterial bubbles, we now suggest the biophysical model presented herein. There are two phases of bubble expansion after decompression. The first is an extended initiation phase, during which nanobubbles are transformed into gas micronuclei and begin to expand. The second, shorter phase is one of simple diffusion-driven growth, the inert gas tension in the blood remaining almost constant during bubble expansion. Detachment of the bubble occurs when its buoyancy exceeds the intermembrane force. Three mechanisms underlying the appearance of arterial bubbles should be considered: patent foramen ovale, intrapulmonary arteriovenous anastomoses, and the evolution of bubbles in the distal arteries with preference for the spinal cord. Other parameters that may be quantified include age, acclimation, distribution of bubble volume, AHS, individual sensitivity, and frequency of bubble formation. We believe that the vascular bubble model we propose adheres more closely to proven physiological processes. Its predictability may therefore be higher than other models, with appropriate adjustments for decompression illness (DCI) data.
Keywords: Active hydrophobic spot; arterial bubbles; bubble expansion; decompression illness.
© 2017 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of The Physiological Society and the American Physiological Society.
Figures




Similar articles
-
Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease-An Overview.Front Physiol. 2017 Aug 15;8:591. doi: 10.3389/fphys.2017.00591. eCollection 2017. Front Physiol. 2017. PMID: 28861003 Free PMC article. Review.
-
DCS or DCI? The difference and why it matters.Diving Hyperb Med. 2019 Sep 30;49(3):152-153. doi: 10.28920/dhm49.3.152-153. Diving Hyperb Med. 2019. PMID: 31523788 Free PMC article.
-
Gas micronuclei that underlie decompression bubbles and decompression sickness have not been identified.Diving Hyperb Med. 2019 Mar 31;49(1):64. doi: 10.28920/dhm49.1.64. Diving Hyperb Med. 2019. PMID: 30856670 Free PMC article.
-
Decompression sickness, fatness and active hydrophobic spots.Diving Hyperb Med. 2018 Sep 30;48(3):130-131. doi: 10.28920/dhm48.3.130-131. Diving Hyperb Med. 2018. PMID: 30199886 Free PMC article.
-
Taravana, vestibular decompression illness, and autochthonous distal arterial bubbles.Respir Physiol Neurobiol. 2019 Jan;259:119-121. doi: 10.1016/j.resp.2018.08.010. Epub 2018 Aug 30. Respir Physiol Neurobiol. 2019. PMID: 30172778 Review.
Cited by
-
Nanobubbles Form at Active Hydrophobic Spots on the Luminal Aspect of Blood Vessels: Consequences for Decompression Illness in Diving and Possible Implications for Autoimmune Disease-An Overview.Front Physiol. 2017 Aug 15;8:591. doi: 10.3389/fphys.2017.00591. eCollection 2017. Front Physiol. 2017. PMID: 28861003 Free PMC article. Review.
-
Taravana syndrome and posterior reversible encephalopathy syndrome: a microbubble hypothesis for neurological accidents in breath-hold divers.Front Physiol. 2024 Sep 24;15:1478650. doi: 10.3389/fphys.2024.1478650. eCollection 2024. Front Physiol. 2024. PMID: 39381329 Free PMC article.
-
In vitro evidence of decompression bubble dynamics and gas exchange on the luminal aspect of blood vessels: Implications for size distribution of venous bubbles.Physiol Rep. 2019 Dec;7(24):e14317. doi: 10.14814/phy2.14317. Physiol Rep. 2019. PMID: 31876064 Free PMC article.
-
High Bubble Grade After Diving: The Role of the Blood Pressure Regimen.Front Physiol. 2019 Jun 20;10:749. doi: 10.3389/fphys.2019.00749. eCollection 2019. Front Physiol. 2019. PMID: 31281261 Free PMC article.
-
Transit Time Measurement in Indicator Dilution Curves: Overcoming the Missing Ground Truth and Quantifying the Error.Front Physiol. 2021 May 28;12:588120. doi: 10.3389/fphys.2021.588120. eCollection 2021. Front Physiol. 2021. PMID: 34122123 Free PMC article.
References
-
- Ackroyd, N. , Gill R., Griffiths K., Kossoff G., and Appleberg M.. 1986. Quantitative common carotid artery blood flow: prediction of internal carotid artery stenosis. J. Vasc. Surg. 3:846–853. - PubMed
-
- Alastruey, J. , Parker K. H., Peiró J., Byrd S. M., and Sherwin S. J.. 2007. Modelling the circle of Willis to assess the effects of anatomical variations and occlusions on cerebral flows. J. Biomech. 40:1794–1805. - PubMed
-
- Arieli, R. 2015. Was the appearance of surfactants in air breathing vertebrates ultimately the cause of decompression sickness and autoimmune disease? Respir. Physiol. Neurobiol. 206:15–18. - PubMed
-
- Arieli, R. , and Marmur A.. 2011. Decompression sickness bubbles: are gas micronuclei formed on a flat hydrophobic surface? Respir. Physiol. Neurobiol. 177:19–23. - PubMed
-
- Arieli, R. , and Marmur A.. 2013a. Dynamics of gas micronuclei formed on a flat hydrophobic surface, the predecessors of decompression bubbles. Respir. Physiol. Neurobiol. 185:647–652. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

