Recurrent rewiring and emergence of RNA regulatory networks
- PMID: 28320951
- PMCID: PMC5389312
- DOI: 10.1073/pnas.1617777114
Recurrent rewiring and emergence of RNA regulatory networks
Abstract
Alterations in regulatory networks contribute to evolutionary change. Transcriptional networks are reconfigured by changes in the binding specificity of transcription factors and their cognate sites. The evolution of RNA-protein regulatory networks is far less understood. The PUF (Pumilio and FBF) family of RNA regulatory proteins controls the translation, stability, and movements of hundreds of mRNAs in a single species. We probe the evolution of PUF-RNA networks by direct identification of the mRNAs bound to PUF proteins in budding and filamentous fungi and by computational analyses of orthologous RNAs from 62 fungal species. Our findings reveal that PUF proteins gain and lose mRNAs with related and emergent biological functions during evolution. We demonstrate at least two independent rewiring events for PUF3 orthologs, independent but convergent evolution of PUF4/5 binding specificity and the rewiring of the PUF4/5 regulons in different fungal lineages. These findings demonstrate plasticity in RNA regulatory networks and suggest ways in which their rewiring occurs.
Keywords: 3′UTR elements; PUF proteins; RNA regulation; evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
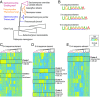

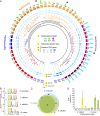



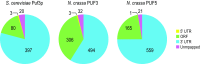


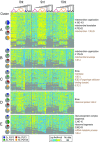
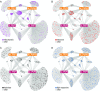
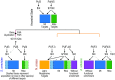
Comment in
-
Reply to Hogan: Direct evidence of RNA-protein interactions and rewiring.Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):E10854-E10855. doi: 10.1073/pnas.1717585114. Epub 2017 Dec 5. Proc Natl Acad Sci U S A. 2017. PMID: 29208722 Free PMC article. No abstract available.
-
Clarifying results and prevailing models for the evolution of Puf proteins and their RNA targets.Proc Natl Acad Sci U S A. 2017 Dec 19;114(51):E10853. doi: 10.1073/pnas.1716625114. Epub 2017 Dec 5. Proc Natl Acad Sci U S A. 2017. PMID: 29208723 Free PMC article. No abstract available.
References
-
- Whiteway M, Tebung WA, Choudhury BI, Rodríguez-Ortiz R. Metabolic regulation in model ascomycetes--Adjusting similar genomes to different lifestyles. Trends Genet. 2015;31(8):445–453. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

