Effective combinatorial immunotherapy for castration-resistant prostate cancer
- PMID: 28321130
- PMCID: PMC5374023
- DOI: 10.1038/nature21676
Effective combinatorial immunotherapy for castration-resistant prostate cancer
Erratum in
-
Erratum: Effective combinatorial immunotherapy for castration-resistant prostate cancer.Nature. 2017 May 3;545(7652):116. doi: 10.1038/nature22348. Nature. 2017. PMID: 28470201 No abstract available.
Abstract
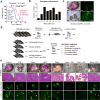
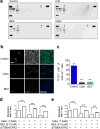
A significant fraction of patients with advanced prostate cancer treated with androgen deprivation therapy experience relapse with relentless progression to lethal metastatic castration-resistant prostate cancer (mCRPC). Immune checkpoint blockade using antibodies against cytotoxic-T-lymphocyte-associated protein 4 (CTLA4) or programmed cell death 1/programmed cell death 1 ligand 1 (PD1/PD-L1) generates durable therapeutic responses in a significant subset of patients across a variety of cancer types. However, mCRPC showed overwhelming de novo resistance to immune checkpoint blockade, motivating a search for targeted therapies that overcome this resistance. Myeloid-derived suppressor cells (MDSCs) are known to play important roles in tumour immune evasion. The abundance of circulating MDSCs correlates with prostate-specific antigen levels and metastasis in patients with prostate cancer. Mouse models of prostate cancer show that MDSCs (CD11b+Gr1+) promote tumour initiation and progression. These observations prompted us to hypothesize that robust immunotherapy responses in mCRPC may be elicited by the combined actions of immune checkpoint blockade agents together with targeted agents that neutralize MDSCs yet preserve T-cell function. Here we develop a novel chimaeric mouse model of mCRPC to efficiently test combination therapies in an autochthonous setting. Combination of anti-CTLA4 and anti-PD1 engendered only modest efficacy. Targeted therapy against mCRPC-infiltrating MDSCs, using multikinase inhibitors such as cabozantinib and BEZ235, also showed minimal anti-tumour activities. Strikingly, primary and metastatic CRPC showed robust synergistic responses when immune checkpoint blockade was combined with MDSC-targeted therapy. Mechanistically, combination therapy efficacy stemmed from the upregulation of interleukin-1 receptor antagonist and suppression of MDSC-promoting cytokines secreted by prostate cancer cells. These observations illuminate a clinical path hypothesis for combining immune checkpoint blockade with MDSC-targeted therapies in the treatment of mCRPC.
Conflict of interest statement
Figures











Comment in
-
Prostate cancer: New model to test immunotherapy combinations.Nat Rev Urol. 2017 Jun;14(6):323. doi: 10.1038/nrurol.2017.44. Epub 2017 Mar 21. Nat Rev Urol. 2017. PMID: 28322260 No abstract available.
-
Re: Effective Combinatorial Immunotherapy for Castration-Resistant Prostate Cancer.J Urol. 2017 Nov;198(5):982-983. doi: 10.1016/j.juro.2017.08.037. Epub 2017 Aug 9. J Urol. 2017. PMID: 29059776 No abstract available.
References
-
- Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348:56–61. - PubMed
-
- Beer TM, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. Journal of Clinical Oncology. 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

