The Antimalarial Chloroquine Suppresses LPS-Induced NLRP3 Inflammasome Activation and Confers Protection against Murine Endotoxic Shock
- PMID: 28321151
- PMCID: PMC5340938
- DOI: 10.1155/2017/6543237
The Antimalarial Chloroquine Suppresses LPS-Induced NLRP3 Inflammasome Activation and Confers Protection against Murine Endotoxic Shock
Abstract
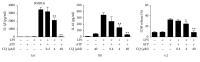
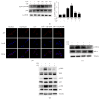
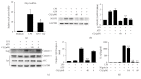
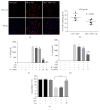
Activation of the NLRP3 inflammasome, which catalyzes maturation of proinflammatory cytokines like IL-1β and IL-18, is implicated and essentially involved in many kinds of inflammatory disorders. Chloroquine (CQ) is a traditional antimalarial drug and also possesses an anti-inflammatory property. In this study, we investigated whether CQ suppresses NLRP3 inflammasome activation and thereby confers protection against murine endotoxic shock. CQ attenuated NF-κB and MAPK activation and prohibited expression of IL-1β, IL-18, and Nlrp3 in LPS treated murine bone marrow-derived macrophages (BMDMs), demonstrating its inhibitory effect on the priming signal of NLRP3 activation. Then, CQ was shown to inhibit caspase-1 activation and ASC specks formation in BMDMs, which indicates that CQ also suppresses inflammasome assembly, the second signal for NLRP3 inflammasome activation. In a murine endotoxic shock model, CQ effectively improved survival and markedly reduced IL-1β and IL-18 production in serum, peritoneal fluid, and lung tissues. Moreover, CQ reduced protein levels of NLRP3 and caspases-1 p10 in lung homogenates of mice with endotoxic shock, which may possibly explain its anti-inflammatory activity and life protection efficacy in vivo. Overall, our results demonstrate a new role of CQ that facilitates negative regulation on NLRP3 inflammasome, which thereby confers protection against lethal endotoxic shock.
Conflict of interest statement
The authors declare that there are no competing interests regarding the publication of this paper.
Figures


References
-
- Grellier N., Deray G., Yousfi A., Khodari W., Bouaita R., Belkacemi Y. Carence martiale fonctionnelle, inflammation et fatigue après radiothérapie. Bulletin Du Cancer. 2015;102(9):780–785. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

