Challenges with targeted viral load testing for medical inpatients at Queen Elizabeth Central Hospital in Blantyre, Malawi
- PMID: 28321282
- PMCID: PMC5348611
- DOI: 10.4314/mmj.v28i4.6
Challenges with targeted viral load testing for medical inpatients at Queen Elizabeth Central Hospital in Blantyre, Malawi
Abstract
Background: Approximately 75% of medical inpatients at Queen Elizabeth Central Hospital (QECH) in Blantyre, Malawi are HIV seropositive, and a third of these patients are on antiretroviral therapy (ART). Malawi guidelines recommend targeted viral load (VL) testing for patients on ART for at least one year who report excellent adherence and present with a WHO clinical stage 3 or 4 HIV disease. A switch to second-line ART is only indicated if a VL result >5000 copies/mL confirms treatment failure.
Methods: During an audit of targeted VL testing at QECH, all adult medical admissions were screened to identify those in need of VL testing. Daily review of inpatient notes ascertained whether VL testing was ordered and carried out. At 8 weeks post-discharge the laboratory database was checked for results and was triangulated with the HIV outpatient database to ascertain whether patients had attended clinic, received results, and if these results had been acted upon.
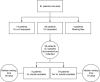
Results: Out of 81 patients recruited, 63 (77%) had a VL requested. At 8 weeks post-discharge, nine patients (14%) had VL results available. The median (IQR) waiting time for those with results was 29 days (20-47). Five patients had a VL >5000 copies/mL. Of these patients, three attended clinic and one was switched to second-line ART. Of the remaining 55 patients awaiting results, the median (IQR) waiting time at the 8-week follow-up point was 72 days (67-80). At 8 weeks post-discharge, 8 patients (33%) had died.
Conclusions: Our findings demonstrate challenges with targeted VL testing at QECH. Only two-thirds of patients with clinical ART failure were identified as eligible for targeted VL testing, and of these less than one-sixth had VL results available after 8 weeks. Interventions such as point-of-care targeted VL testing could result in faster turnaround times. In the interim, we suggest further evaluation of the possibility of switching patients with clinical ART failure and a low CD4 count to second-line ART while awaiting VL results.
Figures
Similar articles
-
Point-of-care viral load monitoring: outcomes from a decentralized HIV programme in Malawi.J Int AIDS Soc. 2019 Aug;22(8):e25387. doi: 10.1002/jia2.25387. J Int AIDS Soc. 2019. PMID: 31441242 Free PMC article.
-
Dried blood spots for viral load monitoring in Malawi: feasible and effective.PLoS One. 2015 Apr 21;10(4):e0124748. doi: 10.1371/journal.pone.0124748. eCollection 2015. PLoS One. 2015. PMID: 25898365 Free PMC article.
-
Virological outcomes of antiretroviral therapy in Zomba central prison, Malawi; a cross-sectional study.J Int AIDS Soc. 2017 Aug 2;20(1):21623. doi: 10.7448/IAS.20.1.21623. J Int AIDS Soc. 2017. PMID: 28782332 Free PMC article.
-
Viral load monitoring for people living with HIV in the era of test and treat: progress made and challenges ahead - a systematic review.BMC Public Health. 2022 Jun 16;22(1):1203. doi: 10.1186/s12889-022-13504-2. BMC Public Health. 2022. PMID: 35710413 Free PMC article.
-
Advances in biosensing strategies for HIV-1 detection, diagnosis, and therapeutic monitoring.Adv Drug Deliv Rev. 2016 Aug 1;103:90-104. doi: 10.1016/j.addr.2016.05.018. Epub 2016 Jun 2. Adv Drug Deliv Rev. 2016. PMID: 27262924 Free PMC article. Review.
Cited by
-
Point-of-care viral load tests to detect high HIV viral load in people living with HIV/AIDS attending health facilities.Cochrane Database Syst Rev. 2022 Mar 10;3(3):CD013208. doi: 10.1002/14651858.CD013208.pub2. Cochrane Database Syst Rev. 2022. PMID: 35266555 Free PMC article.
-
Point-of-Care HIV Viral Load Testing: an Essential Tool for a Sustainable Global HIV/AIDS Response.Clin Microbiol Rev. 2019 May 15;32(3):e00097-18. doi: 10.1128/CMR.00097-18. Print 2019 Jun 19. Clin Microbiol Rev. 2019. PMID: 31092508 Free PMC article. Review.
-
Virological failure, HIV-1 drug resistance, and early mortality in adults admitted to hospital in Malawi: an observational cohort study.Lancet HIV. 2020 Sep;7(9):e620-e628. doi: 10.1016/S2352-3018(20)30172-7. Lancet HIV. 2020. PMID: 32890497 Free PMC article.
-
Advanced HIV disease management practices within inpatient medicine units at a referral hospital in Zambia: a retrospective chart review.AIDS Res Ther. 2022 Feb 22;19(1):10. doi: 10.1186/s12981-022-00433-8. AIDS Res Ther. 2022. PMID: 35193598 Free PMC article.
-
Tuberculosis in Hospitalized Patients With Human Immunodeficiency Virus: Clinical Characteristics, Mortality, and Implications From the Rapid Urine-based Screening for Tuberculosis to Reduce AIDS Related Mortality in Hospitalized Patients in Africa.Clin Infect Dis. 2020 Dec 17;71(10):2618-2626. doi: 10.1093/cid/ciz1133. Clin Infect Dis. 2020. PMID: 31781758 Free PMC article.
References
-
- Ministry of Health, Malawi, author. Ministry of Health, Malawi. Clinical management of HIV in children and adults [Internet] Lilongwe: Ministry of Health, Malawi; 2014. Feb, [cited 2016 Mar 30]. 95 p. Available from: http://www.emtct-iatt.org/wp-content/uploads/2015/09/Malawi-HIV-Guidelin....
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials