Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth
- PMID: 28321847
- PMCID: PMC6464568
- DOI: 10.1002/14651858.CD004454.pub3
Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth
Update in
-
Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth.Cochrane Database Syst Rev. 2020 Dec 25;12(12):CD004454. doi: 10.1002/14651858.CD004454.pub4. Cochrane Database Syst Rev. 2020. PMID: 33368142 Free PMC article.
Abstract
Background: Respiratory morbidity including respiratory distress syndrome (RDS) is a serious complication of preterm birth and the primary cause of early neonatal mortality and disability. While researching the effects of the steroid dexamethasone on premature parturition in fetal sheep in 1969, Liggins found that there was some inflation of the lungs of lambs born at gestations at which the lungs would be expected to be airless. Liggins and Howie published the first randomised controlled trial in humans in 1972 and many others followed.
Objectives: To assess the effects of administering a course of corticosteroids to the mother prior to anticipated preterm birth on fetal and neonatal morbidity and mortality, maternal mortality and morbidity, and on the child in later life.
Search methods: We searched Cochrane Pregnancy and Childbirth's Trials Register (17 February 2016) and reference lists of retrieved studies.
Selection criteria: We considered all randomised controlled comparisons of antenatal corticosteroid administration (betamethasone, dexamethasone, or hydrocortisone) with placebo, or with no treatment, given to women with a singleton or multiple pregnancy, prior to anticipated preterm delivery (elective, or following spontaneous labour), regardless of other co-morbidity, for inclusion in this review. Most women in this review received a single course of steroids; however, nine of the included trials allowed for women to have weekly repeats.
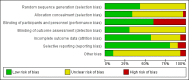
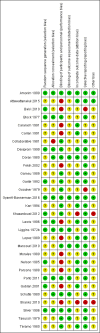
Data collection and analysis: Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. The quality of the evidence was assessed using the GRADE approach.
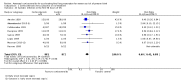
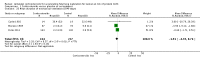
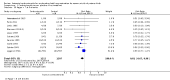
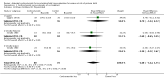
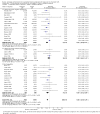
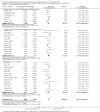
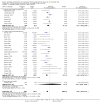
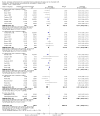
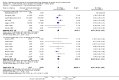
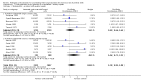
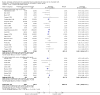
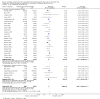
Main results: This update includes 30 studies (7774 women and 8158 infants). Most studies are of low or unclear risk for most bias domains. An assessment of high risk usually meant a trial had potential for performance bias due to lack of blinding. Two trials had low risks of bias for all risk of bias domains.Treatment with antenatal corticosteroids (compared with placebo or no treatment) is associated with a reduction in the most serious adverse outcomes related to prematurity, including: perinatal death (average risk ratio (RR) 0.72, 95% confidence interval (CI) 0.58 to 0.89; participants = 6729; studies = 15; Tau² = 0.05, I² = 34%; moderate-quality); neonatal death (RR 0.69, 95% CI 0.59 to 0.81; participants = 7188; studies = 22), RDS (average RR 0.66, 95% CI 0.56 to 0.77; participants = 7764; studies = 28; Tau² = 0.06, I² = 48%; moderate-quality); moderate/severe RDS (average RR 0.59, 95% CI 0.38 to 0.91; participants = 1686; studies = 6; Tau² = 0.14, I² = 52%); intraventricular haemorrhage (IVH) (average RR 0.55, 95% CI 0.40 to 0.76; participants = 6093; studies = 16; Tau² = 0.10, I² = 33%; moderate-quality), necrotising enterocolitis (RR 0.50, 95% CI 0.32 to 0.78; participants = 4702; studies = 10); need for mechanical ventilation (RR 0.68, 95% CI 0.56 to 0.84; participants = 1368; studies = 9); and systemic infections in the first 48 hours of life (RR 0.60, 95% CI 0.41 to 0.88; participants = 1753; studies = 8).There was no obvious benefit for: chronic lung disease (average RR 0.86, 95% CI 0.42 to 1.79; participants = 818; studies = 6; Tau² = 0.38 I² = 65%); mean birthweight (g) (MD -18.47, 95% CI -40.83 to 3.90; participants = 6182; studies = 16; moderate-quality); death in childhood (RR 0.68, 95% CI 0.36 to 1.27; participants = 1010; studies = 4); neurodevelopment delay in childhood (RR 0.64, 95% CI 0.14 to 2.98; participants = 82; studies = 1); or death into adulthood (RR 1.00, 95% CI 0.56 to 1.81; participants = 988; studies = 1).Treatment with antenatal corticosteroids does not increase the risk of chorioamnionitis (RR 0.83, 95% CI 0.66 to 1.06; participants = 5546; studies = 15; moderate-quality evidence) or endometritis (RR 1.20, 95% CI 0.87 to 1.63; participants = 4030; studies = 10; Tau² = 0.11, I² = 28%; moderate-quality). No increased risk in maternal death was observed. However, the data on maternal death is based on data from a single trial with two deaths; four other trials reporting maternal death had zero events (participants = 3392; studies = 5; moderate-quality).There is no definitive evidence to suggest that antenatal corticosteroids work differently in any pre-specified subgroups (singleton versus multiple pregnancy; membrane status; presence of hypertension) or for different study protocols (type of corticosteroid; single course or weekly repeats).GRADE outcomes were downgraded to moderate-quality. Downgrading decisions (for perinatal death, RDS, IVH, and mean birthweight) were due to limitations in study design or concerns regarding precision (chorioamnionitis, endometritis). Maternal death was downgraded for imprecision due to few events.
Authors' conclusions: Evidence from this update supports the continued use of a single course of antenatal corticosteroids to accelerate fetal lung maturation in women at risk of preterm birth. A single course of antenatal corticosteroids could be considered routine for preterm delivery. It is important to note that most of the evidence comes from high income countries and hospital settings; therefore, the results may not be applicable to low-resource settings with high rates of infections.There is little need for further trials of a single course of antenatal corticosteroids versus placebo in singleton pregnancies in higher income countries and hospital settings. However, data are sparse in lower income settings. There are also few data regarding risks and benefits of antenatal corticosteroids in multiple pregnancies and other high-risk obstetric groups. Further information is also required concerning the optimal dose-to-delivery interval, and the optimal corticosteroid to use.We encourage authors of previous studies to provide further information, which may answer any remaining questions about the use of antenatal corticosteroids in such pregnancies without the need for further randomised controlled trials. Individual patient data meta-analysis from published trials is likely to answer some of the evidence gaps. Follow-up studies into childhood and adulthood, particularly in the late preterm gestation and repeat courses groups, are needed. We have not examined the possible harmful effects of antenatal corticosteroids in low-resource settings in this review. It would be particularly relevant to explore this finding in adequately powered prospective trials.
Conflict of interest statement
Devender Roberts: none known.
Julie Brown: none known.
Nancy Medley: Nancy Medley's work was financially supported by the a grant to University of Liverpool from the Harris‐Wellbeing Preterm Birth Centre.
Stuart R Dalziel: none known.
Figures























































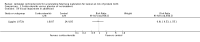








































































Update of
-
Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth.Cochrane Database Syst Rev. 2006 Jul 19;(3):CD004454. doi: 10.1002/14651858.CD004454.pub2. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Mar 21;3:CD004454. doi: 10.1002/14651858.CD004454.pub3. PMID: 16856047 Updated.
Comment in
-
Corticosteroids for preterm deliveries: missing evidence.Cochrane Database Syst Rev. 2017 May 3;5(5):ED000121. doi: 10.1002/14651858.ED000121. Cochrane Database Syst Rev. 2017. PMID: 28497861 Free PMC article. No abstract available.
-
The profile of a life-saving researcher.Educ Prim Care. 2018 Mar;29(2):113-114. doi: 10.1080/14739879.2018.1437570. Epub 2018 Feb 15. Educ Prim Care. 2018. PMID: 29447583
References
References to studies included in this review
-
- Amorim MM, Santos LC, Faundes A. Corticosteroid therapy for prevention of respiratory distress syndrome in severe preeclampsia. American Journal of Obstetrics and Gynecology 1999;180(5):1283‐8. - PubMed
-
- Attawattanakul N, Tansupswatdikul P. Effects of antenatal dexamethasone on respiratory distress in late preterm infant: a randomized controlled trial. Thai Journal of Obstetrics and Gynaecology 2015;23:25‐33.
-
- Balci O, Ozdemir S, Mahmoud AS, Acar A, Colakoglu MC. The effect of antenatal steroids on fetal lung maturation between the 34th and 36th week of pregnancy. Gynecologic and Obstetric Investigation 2010;70(2):95‐9. - PubMed
-
- Block MF, Kling OR, Crosby WM. Antenatal glucocorticoid therapy for the prevention of respiratory distress syndrome in the premature infant. Obstetrics & Gynecology 1977;50:186‐90. - PubMed
-
- Botet F, Cararach V, Sentis J. Premature rupture of membranes in early pregnancy. Neonatal prognosis. Journal of Perinatal Medicine 1994;22:45‐52. - PubMed
- Cararach V, Botet F, Sentis J, Carmona F. A multicenter, prospective randomized study in premature rupture of membranes (PROM). Maternal and perinatal complications. International Journal of Gynecology and Obstetrics 1991;36 Suppl:267.
- Cararach V, Sentis J, Botet F, Rios L. A multicenter, prospective randomized study in premature rupture of membranes (PROM). Respiratory and infectious complications in the newborn. Proceedings of the 12th European Congress of Perinatal Medicine; 1990; Lyon, France. 1990:216.
References to studies excluded from this review
-
- Abuhamad A, Green G, Heyl P, Veciana M. The combined use of corticosteroids and thyrotropin releasing hormone in pregnancies with preterm rupture of membranes: a randomised double blind controlled trial. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S96.
-
- Althabe F, Belizan JM, Mazzoni A, Berrueta M, Hemingway‐Foday J, Koso‐Thomas M, et al. Antenatal corticosteroids trial in preterm births to increase neonatal survival in developing countries: study protocol. Reproductive Health 2012;9(1):22. - PMC - PubMed
- Althabe F, Belizan JM, McClure EM, Hemingway‐Foday J, Berrueta M, Mazzoni A, et al. A population‐based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low‐income and middle‐income countries: the ACT cluster‐randomised trial. Lancet 2014;385(9968):629‐639. [PUBMED: 25458726] - PMC - PubMed
-
- Asnafei N, Pourreza R, Miri SM. Pregnancy outcome in premature delivery of between 34‐37 weeks and the effects of corticosteroid on it. Journal of the Gorgan University of Medical Sciences 2004;6(2):57‐60.
-
- Butterfill AM, Harvey DR. Follow‐up study of babies exposed to betamethasone before birth. Archives of Disease in Childhood 1979;54:725.
-
- Dola C, Nageotte M, Rumney P, Towers C, Asrat T, Freeman R, et al. The effect of antenatal treatment with betamethasone and thyrotropin releasing hormone in patients with preterm premature rupture of membranes. American Journal of Obstetrics and Gynecology 1997;176(1 Pt 2):S49.
Additional references
-
- Althabe F, Belizan J, McClure E, Hemingway‐Foday J, Berrueta M, Mazzoni A, et al. A population‐based, multifaceted strategy to implement antenatal corticosteroid treatment versus standard care for the reduction of neonatal mortality due to preterm birth in low‐income and middle‐income countries: the ACT cluster‐ randomised trial. The Lancet 2014;385(9968):629‐39. [DOI: 10.1016/S0140-6736(14)61651-2] - DOI - PMC - PubMed
-
- Amiya RM, Mlunde LB, Ota E, Swa T, Oladapo O, Mori R. Antenatal corticosteroids for reducing adverse maternal and child outcomes in special populations of women at risk of imminent preterm birth: a systematic review and meta‐analysis. PLoS One 2016;11(2):e0147604. [DOI: 10.1371/journal.pone.0147604] - DOI - PMC - PubMed
-
- Antenatal Corticosteroids Clinical Practice Guidelines Panel. Antenatal corticosteroids given to women prior to birth to improve fetal, infant, child and adult health: Clinical Practice Guidelines. Liggins Institute, The University of Auckland, Auckland, New Zealand2015.
-
- Barker DJP. Mothers, Babies and Health in Later Life. 2nd Edition. London: Churchill Livingstone, 1998.
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

