Pharmacological interventions for acute hepatitis B infection: an attempted network meta-analysis
- PMID: 28321877
- PMCID: PMC6464625
- DOI: 10.1002/14651858.CD011645.pub2
Pharmacological interventions for acute hepatitis B infection: an attempted network meta-analysis
Abstract
Background: Infection with hepatitis B virus (HBV) can be symptomatic or asymptomatic. Apart from chronic HBV infection, the complications related to acute HBV infection are severe acute viral hepatitis and fulminant hepatitis characterised by liver failure. The optimal pharmacological treatment of acute HBV infection remains controversial.
Objectives: To assess the benefits and harms of pharmacological interventions in the treatment of acute HBV infection through a network meta-analysis and to generate rankings of the available treatments according to their safety and efficacy. As it was not possible to assess whether the potential effect modifiers were similar across different comparisons, we did not perform the network meta-analysis, and instead, assessed the benefits and harms of different interventions using standard Cochrane methodological procedures.
Search methods: We searched CENTRAL, MEDLINE, Embase, Science Citation Index Expanded, WHO International Clinical Trials Registry Platform, and randomised clinical trials (RCTs) registers to August 2016 to identify RCTs on pharmacological interventions for acute HBV infection.
Selection criteria: RCTs, irrespective of language, blinding, or publication status in participants with acute HBV infection. We excluded trials if participants had previously undergone liver transplantation and had other coexisting viral diseases such as hepatitis C virus and HIV. We considered any of the various pharmacological interventions compared with each other or with placebo, or no intervention.
Data collection and analysis: We calculated the odds ratio (OR) and rate ratio with 95% confidence intervals (CI) using both fixed-effect and random-effects models based on available-participant analysis with Review Manager 5. We assessed risk of bias, controlled risk of random errors with Trial Sequential Analysis, and assessed the quality of the evidence using GRADE.
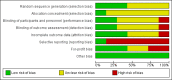
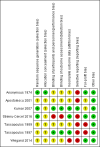
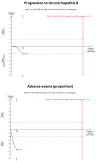
Main results: Seven trials (597 participants) met our review inclusion criteria. All trials provided information for one or more outcomes; however, five participants were excluded from analysis by study authors. All the trials were at high risk of bias. Overall, all the evidence was low or very low quality evidence because of risk of bias (downgraded one level for risk of bias), small sample size (downgraded one level for imprecision), and wide CIs (downgraded one more level for imprecision in some comparisons). Of the seven trials, six were two-armed trials, while one trial was a three-armed trial. The comparisons included hepatitis B immunoglobulin (HBIG) versus placebo (one trial; 55 participants); interferon versus placebo (two trials; 200 participants); lamivudine versus placebo or no intervention (four trials; 316 participants); lamivudine versus entecavir (one trial; 90 participants); and entecavir versus no intervention (one trial; 131 participants). One trial included only people with acute HBV with hepatic encephalopathy (i.e. people with fulminant liver failure); one trial included only people with severe acute HBV, but it did not state whether any of the people also had fulminant HBV infection; three trials excluded fulminant HBV infection; and two trials did not report the severity of acute HBV infection. The mean or median follow-up period in the trials ranged from three to 12 months in the trials that provided this information.There was no evidence of any differences in short-term mortality (less than one year) in any of the comparisons: HBIG versus placebo (OR 1.13, 95% CI 0.36 to 3.54; participants = 55; 1 trial), lamivudine versus placebo or no intervention (OR 1.29, 95% CI 0.33 to 4.99; participants = 250; 2 trials); lamivudine versus entecavir (OR 1.23, 95% CI 0.13 to 11.65; participants = 90; 1 trial), or entecavir versus no intervention (OR 1.05, 95% CI 0.12 to 9.47; participants = 131; 1 trial). The proportion of people who progressed to chronic HBV infection was higher in the lamivudine group than the placebo or no intervention group (OR 1.99, 95% CI 1.05 to 3.77; participants = 285; 3 trials) and in the lamivudine group versus entecavir group (OR 3.64, 95% CI 1.31 to 10.13; participants = 90; 1 trial). There was no evidence of a difference in the proportion of people who progressed to chronic HBV infection between the entecavir and the no intervention groups (OR 0.58, 95% CI 0.23 to 1.49; participants = 131; 1 trial). None of the trials reported progression to fulminant HBV infection. Three trials with 371 participants reported serious adverse events. There were no serious adverse events in any of the groups (no intervention: 0/183 (0%), interferon: 0/67 (0%), lamivudine: 0/100 (0%), and entecavir: 0/21 (0%)). The proportion of people with adverse events was higher in the interferon group than the placebo group (OR 348.16, 95% CI 45.39 to 2670.26; participants = 200; 2 trials). There was no evidence of a difference in the proportion of people with adverse events between the lamivudine group and the placebo or no intervention group (OR 1.42, 95% CI 0.34 to 5.94; participants = 35; 1 trial) or number of adverse events between the lamivudine group and the placebo or no intervention group (rate ratio 1.72, 95% CI 1.01 to 2.91; participants = 35; 1 trial). One trial with 100 participants reported quality of life at one week. The scale used to report the health-related quality of life was not stated and lacked information on whether higher score meant better or worse, making it difficult to interpret the results. None of the trials reported quality of life beyond one week or other clinical outcomes such as mortality beyond one year, liver transplantation, cirrhosis, decompensated cirrhosis, or hepatocellular carcinoma.Two trials received funding from pharmaceutical companies; three trials were funded by parties without any vested interest in the results or did not receive any special funding; the source of funding was not available in the remaining two trials.
Authors' conclusions: Low or very low quality evidence suggests that progression to chronic HBV infection was higher in people receiving lamivudine compared with placebo, no intervention, or entecavir. Low quality evidence suggests that interferon may increase the adverse events after treatment for acute HBV infection. Based on a very low quality evidence, there is currently no evidence of benefit of any intervention in acute HBV infection. There is significant uncertainty in the results and further RCTs are required.
Conflict of interest statement
This report is independent research funded by the National Institute for Health Research (NIHR Cochrane Programme Grants, 13/89/03 ‐ evidence‐based diagnosis and management of upper digestive, hepato‐biliary, and pancreatic disorders). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service (NHS), the NIHR, or the Department of Health.
KM: no financial disclosures. MRP: acted as advisory for Astellas and has received educational grants from Astellas and Novartis. EB: no financial disclosures. DT: funded by Astellas for his attendance at the International Liver Transplantation Society meeting in 2014; received GBP 25,000 from Boston Scientific to fund a clinical research fellow in 2013. BD: no financial disclosures. ET: no financial disclosures. KG: no financial disclosures.
Figures




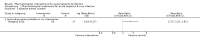


Update of
- doi: 10.1002/14651858.CD011645
Similar articles
-
Pharmacological interventions for acute hepatitis C infection: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 13;3(3):CD011644. doi: 10.1002/14651858.CD011644.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2018 Dec 03;12:CD011644. doi: 10.1002/14651858.CD011644.pub3. PMID: 28285495 Free PMC article. Updated.
-
Pharmacological interventions for primary biliary cholangitis: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 28;3(3):CD011648. doi: 10.1002/14651858.CD011648.pub2. Cochrane Database Syst Rev. 2017. PMID: 28350426 Free PMC article.
-
Management of people with early- or very early-stage hepatocellular carcinoma: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 28;3(3):CD011650. doi: 10.1002/14651858.CD011650.pub2. Cochrane Database Syst Rev. 2017. PMID: 28351116 Free PMC article.
-
Pharmacological interventions for non-alcohol related fatty liver disease (NAFLD): an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 30;3(3):CD011640. doi: 10.1002/14651858.CD011640.pub2. Cochrane Database Syst Rev. 2017. PMID: 28358980 Free PMC article.
-
Interventions for hereditary haemochromatosis: an attempted network meta-analysis.Cochrane Database Syst Rev. 2017 Mar 8;3(3):CD011647. doi: 10.1002/14651858.CD011647.pub2. Cochrane Database Syst Rev. 2017. PMID: 28273330 Free PMC article.
Cited by
-
Epidemiological and clinical aspects of hepatitis B virus infection in Italy over the last 50 years.World J Gastroenterol. 2022 Jul 14;28(26):3081-3091. doi: 10.3748/wjg.v28.i26.3081. World J Gastroenterol. 2022. PMID: 36051347 Free PMC article. Review.
-
Jian Gan powder ameliorates immunological liver injury in mice by modulating the gut microbiota and metabolic profiles.Eur J Med Res. 2024 Apr 20;29(1):240. doi: 10.1186/s40001-024-01827-2. Eur J Med Res. 2024. PMID: 38641655 Free PMC article.
-
Lack of evidence to favor specific preventive interventions in psychosis: a network meta-analysis.World Psychiatry. 2018 Jun;17(2):196-209. doi: 10.1002/wps.20526. World Psychiatry. 2018. PMID: 29856551 Free PMC article.
-
Management of Acute Liver Failure: Update 2022.Semin Liver Dis. 2022 Aug;42(3):362-378. doi: 10.1055/s-0042-1755274. Epub 2022 Aug 24. Semin Liver Dis. 2022. PMID: 36001996 Free PMC article.
-
Entecavir for children and adults with chronic hepatitis B.Cochrane Database Syst Rev. 2025 Apr 22;4(4):CD015536. doi: 10.1002/14651858.CD015536.pub2. Cochrane Database Syst Rev. 2025. PMID: 40260837
References
References to studies included in this review
Anonymous 1974 {published data only}
-
- Anonymous. Treatment of fulminant type B hepatitis with hepatitis B immune globulin a cooperative trial. Gastroenterology 1974;66:752.
Apostolescu 2001 {published data only}
-
- Apostolescu CG, Rebeda I, Coltan G, Iacob SA, Caruntu FA. The efficacy of lamivudine in acute viral hepatitis with hepatitis B virus [abstract]. Journal of Hepatology 2001;34(1):144.
Kumar 2007 {published data only}
-
- Kumar M, Satapathy S, Monga R, Das K, Hissar S, Pande C, et al. A randomized controlled trial of lamivudine to treat acute hepatitis B. Hepatology (Baltimore, Md.) 2007;45(1):97‐101. - PubMed
Streinu‐Cercel 2016 {published data only}
-
- Streinu‐Cercel A, Sandulescu O, Stefan M, Streinu‐Cercel A. Treatment with lamivudine and entecavir in severe acute hepatitis B. Indian Journal of Medical Microbiology 2016;34(2):166‐72. - PubMed
Tassopoulos 1989 {published data only}
-
- Tassopoulos N, Hadziyannis S, Wright G. A randomized double‐blind placebo‐controlled trial of alpha‐interferon in acute type II hepatitis. Hepatology (Baltimore, Md.) 1989;10(4):576.
Tassopoulos 1997 {published data only}
-
- Tassopoulos NC, Koutelou MG, Polychronaki H, Paraloglou‐Ioannides M, Hadziyannis SJ. Recombinant interferon‐alpha therapy for acute hepatitis B: a randomized, double‐blind, placebo‐controlled trial. Journal of Viral Hepatitis 1997;4(6):387‐94. - PubMed
Wiegand 2014 {published data only}
-
- Wiegand J, Wedemeyer H, Franke A, Roessler S, Zeuzem S, Teuber G, et al. Treatment of severe, nonfulminant acute hepatitis B with lamivudine vs placebo: a prospective randomized double‐blinded multicentre trial. Journal of Viral Hepatitis 2014;21(10):744‐50. - PubMed
-
- Wiegand J, Wedemeyer H, Franke A, Stolzel U, Zeuzem S, Teuber G, et al. Treatment of acute hepatitis B with lamivudine vs. placebo: a prospective randomized multicenter trial. Hepatology (Baltimore, Md.) 2012;56:385A‐6A.
References to studies excluded from this review
Blum 1977 {published data only}
-
- Blum AL, Berthet P, Doelle W, Goebell H, Kortum K, Pelloni S, et al. Treatment of acute viral hepatitis with (+)‐cyanidanol‐3. Lancet 1977;2(8049):1153‐5. - PubMed
Botero 1991 {published data only}
-
- Botero RC, Delgado C. Placebo controlled trial of intravenous s‐adenosylmetionine (SAME) in patients with acute hepatitis‐A, hepatitis‐B, and NNB. Hepatology (Baltimore, Md.) 1991;14(4):A199‐200.
Flisiak 2000 {published data only}
-
- Flisiak R, Prokopowicz D. One year follow‐up of patients treated with misoprostol in acute phase of viral hepatitis B. Prostaglandins & Other Lipid Mediators 2000;60(4‐6):161‐5. - PubMed
Gregory 1976 {published data only}
-
- Gregory PB, Knauer CM, Kempson RL, Miller R. Steroid therapy in severe viral hepatitis. A double‐blind, randomized trial of methyl‐prednisolone versus placebo. New England Journal of Medicine 1976;294(13):681‐7. - PubMed
Sharapov 2000 {published data only}
-
- Sharapov M. Treatment with alfa‐2 interferon in children with acute hepatitis B. Gut 2000;47(Suppl III):A287.
Tillmann 2006 {published data only}
-
- Tillmann HL, Hadem J, Leifeld L, Zachou K, Canbay A, Eisenbach C, et al. Safety and efficacy of lamivudine in patients with severe acute or fulminant hepatitis B, a multicenter experience. Journal of Viral Hepatitis 2006;13(4):256‐63. - PubMed
Yu 2010 {published data only}
-
- Yu JW, Sun LJ, Zhao YH, Kang P, Li SC. The study of efficacy of lamivudine in patients with severe acute hepatitis B. Digestive Diseases & Sciences 2010;55(3):775‐83. - PubMed
Additional references
AASLD 2009
-
- Lok ASF, McMahon BJ. AASLD practice guidelines. Chronic hepatitis B: update 2009. www.aasld.org/practiceguidelines/Documents/Bookmarked%20Practice%20Guide... (accessed 13 October 2014).
Anonymous 1981
Bailon 2001
-
- Bailon P, Palleroni A, Schaffer CA, Spence CL, Fung WJ, Porter JE, et al. Rational design of a potent, long‐lasting form of interferon: a 40 kDa branched polyethylene glycol‐conjugated interferon alpha‐2a for the treatment of hepatitis C. Bioconjugate Chemistry 2001;12(2):195‐202. - PubMed
Bass 2013
-
- Bass S, Zook N. Intravenous acetylcysteine for indications other than acetaminophen overdose. American Journal of Health‐System Pharmacy 2013;70(17):1496‐501. - PubMed
Bruno 2014
-
- Bruno R, Carosi G, Coppola N, Gaeta GB, Puoti M, Santantonio T, et al. Recommendations for the management of acute hepatitis B: position paper of the Italian Society for the Study of Infectious and Tropical Diseases (SIMIT). Infection 2014;42(5):811‐5. - PubMed
CDC 2014
-
- Centers for Disease Control and Prevention. Surveillance for Viral Hepatitis ‐ United States, 2014. www.cdc.gov/hepatitis/statistics/2014surveillance/pdfs/2014hepsurveillan... (accessed on 12 February 2017).
Chaimani 2012
-
- Chaimani A, Salanti G. Using network meta‐analysis to evaluate the existence of small‐study effects in a network of interventions. Research Synthesis Methods 2012;3(2):161‐76. - PubMed
Chaimani 2013
Chan 2013
Choi 2011
Chu 2006
-
- Chu CM, Liaw YF. Hepatitis B virus‐related cirrhosis: natural history and treatment. Seminars in Liver Disease 2006;26(2):142‐52. - PubMed
Coppola 2014
-
- Coppola N, Sagnelli C, Pisaturo M, Minichini C, Messina V, Alessio L, et al. Clinical and virological characteristics associated with severe acute hepatitis B. Clinical Microbiology and Infection 2014;20(12):O991‐7. - PubMed
Del Re 2013
DeMets 1987
-
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. - PubMed
Deng 2012
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. - PubMed
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29(7‐8):932‐44. - PubMed
Dias 2012a
-
- Dias S, Sutton AJ, Welton NJ, Ades AE. NICE DSU Technical Support Document 3: heterogeneity: subgroups, meta‐regression, bias and bias‐adjustment, September 2011 (last updated April 2012). www.nicedsu.org.uk/TSD3%20Heterogeneity.final%20report.08.05.12.pdf (accessed 27 March 2014).
Dias 2012b
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 1: introduction to evidence synthesis for decision making, April 2011 (last updated April 2012). www.nicedsu.org.uk/TSD1%20Introduction.final.08.05.12.pdf (accessed 27 March 2014).
Dias 2014a
-
- Dias S, Welton NJ, Sutton AJ, Ades AE. NICE DSU Technical Support Document 2: a generalised linear modelling framework for pair wise and network meta‐analysis of randomised controlled trials, August 2011 (last updated April 2014). www.nicedsu.org.uk/TSD2%20General%20meta%20analysis%20corrected%2015Apri... (accessed 8 October 2014).
Dias 2014b
-
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: inconsistency in networks of evidence based on randomised controlled trials, May 2011 (last updated April 2014). www.nicedsu.org.uk/TSD4%20Inconsistency.final.15April2014.pdf (accessed 8 October 2014). - PubMed
EASL 2012
-
- European Association for Study of Liver. EASL clinical practice guidelines: management of chronic hepatitis B virus infection. Journal of Hepatology 2012;57(1):167‐85. - PubMed
Egger 1997
EuroQol 2014
-
- EuroQol. About EQ‐5D. www.euroqol.org/about‐eq‐5d.html (accessed 8 October 2014).
Feld 2005
-
- Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005;436(7053):967‐72. - PubMed
Ganem 2004
-
- Ganem D, Prince AM. Hepatitis B virus infection ‐ natural history and clinical consequences. New England Journal of Medicine 2004;350(11):1118‐29. - PubMed
Garfein 2004
-
- Garfein RS, Bower WA, Loney CM, Hutin YJ, Xia GL, Jawanda J, et al. Factors associated with fulminant liver failure during an outbreak among injection drug users with acute hepatitis B. Hepatology (Baltimore, Md.) 2004;40(4):865‐73. - PubMed
Gluud 2016
-
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)) 2016, Issue 10. Art. No.: LIVER.
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. - PubMed
Higgins 2011
-
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2012
Hoofnagle 2007
-
- Hoofnagle JH, Doo E, Liang TJ, Fleischer R, Lok ASF. Management of hepatitis B: summary of a clinical research workshop. Hepatology (Baltimore, Md.) 2007;45(4):1056‐75. - PubMed
ICH‐GCP 1997
-
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Pennsylvania: Barnett International/PAREXEL, 1997.
Jakobsen 2014
Kjaergard 2001
-
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. - PubMed
Kumar 2006
-
- Kumar M, Jain S, Sharma BC, Sarin SK. Differentiating acute hepatitis B from the first episode of symptomatic exacerbation of chronic hepatitis B. Digestive Diseases and Sciences 2006;51(3):594‐9. - PubMed
Lazaridis 2001
-
- Lazaridis KN, Gores GJ, Lindor KD. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders'. Journal of Hepatology 2001;35(1):134‐46. - PubMed
Leen 1989
-
- Leen CL, Davison SM, Flegg PJ, Mandal BK. Seven years experience of acute hepatitis B in a regional department of infectious diseases and tropical medicine. Journal of Infection 1989;18(3):257‐63. - PubMed
Li 2010
Lin 2011
-
- Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: recent advances. Journal of Gastroenterology and Hepatology 2011;26(Suppl 1):123‐30. - PubMed
Lu 2006
-
- Lu G, Ades AE. Assessing evidence inconsistency in mixed treatment comparisons. Journal of the American Statistical Association 2006;101(474):447‐59.
Lundh 2017
Macaskill 2001
-
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. - PubMed
Martindale 2011
-
- Sweetman S, editor. Martindale: the complete drug reference (online version), 37th edition, 2011. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference (accessed 23 September 2014).
McMahon 2014
-
- McMahon BJ. Chronic hepatitis B virus infection. Medical Clinics of North America 2014;98(1):39‐54. - PubMed
Mills 2012
-
- Mills EJ, Ioannidis JP, Thorlund K, Schünemann HJ, Puhan MA, Guyatt GH. How to use an article reporting a multiple treatment comparison meta‐analysis. JAMA 2012;308(12):1246‐53. - PubMed
Moher 1998
-
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. - PubMed
NCBI 2014a
-
- NCBI. Interferons, 2014. www.ncbi.nlm.nih.gov/mesh/68007372 (accessed 11 October 2014).
NCBI 2014b
-
- NCBI. Thymosins, 2014. www.ncbi.nlm.nih.gov/mesh/68013947 (accessed 15 October 2014).
Nelson 2011
Newell 1992
-
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. - PubMed
OpenBUGS 3.2.3 [Computer program]
-
- Members of OpenBUGS Project Management Group. OpenBUGS. Version 3.2.3. Members of OpenBUGS Project Management Group, 2014.
Puhan 2014
-
- Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello‐Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta‐analysis. BMJ (Clinical Research Ed.) 2014;349:g5630. - PubMed
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, Cochrane. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, Cochrane, 2014.
Royle 2003
-
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. - PubMed
Salanti 2011
-
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple‐treatment meta‐analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64(2):163‐71. - PubMed
Salanti 2012
-
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3(2):80‐97. - PubMed
Savović 2012a
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessment 2012;16(35):1‐82. - PubMed
Savović 2012b
-
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. - PubMed
Schulz 1995
-
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. - PubMed
Schulz 2010
Severini 1993
-
- Severini TA. Bayesian interval estimates which are also confidence intervals. Journal of the Royal Statistical Society. Series B (Methodological) 1993;55(2):533‐40.
Sharif 2013
-
- Sharif O, Krishnan PV, Thekdi AD, Gordon SC. Acute hepatitis B in an urban tertiary care hospital in the United States: a cohort evaluation. Journal of Clinical Gastroenterology 2013;47(9):e87‐90. - PubMed
Shi 2010
-
- Shi Z, Li X, Ma L, Yang Y. Hepatitis B immunoglobulin injection in pregnancy to interrupt hepatitis B virus mother‐to‐child transmission‐a meta‐analysis. International Journal of Infectious Diseases 2010;14(7):e622‐34. - PubMed
Souza 2013
Stata/SE 14.2 [Computer program]
-
- StataCorp LP. Stata/SE 14.2 for Windows[64‐bit x86‐64]. Version 14. College Station: StataCorp LP, 2017.
Tanwar 2012
-
- Tanwar S, Dusheiko G. Is there any value to hepatitis B virus genotype analysis?. Current Gastroenterology Reports 2012;14(1):37‐46. - PubMed
Thiele 2014
Thompson 2009
-
- Thompson ND, Perz JF, Moorman AC, Holmberg SD. Nonhospital health care‐associated hepatitis B and C virus transmission: United States, 1998‐2008. Annals of Internal Medicine 2009;150(1):33‐9. - PubMed
Thorlund 2011
-
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 24 November 2016).
Thorlund 2012
Tillmann 2012
-
- Tillmann HL, Zachou K, Dalekos GN. Management of severe acute to fulminant hepatitis B: to treat or not to treat or when to treat?. Liver International 2012;32(4):544‐53. - PubMed
Tillmann 2014
-
- Tillmann HL, Patel K. Therapy of acute and fulminant hepatitis B. Intervirology 2014;57(3‐4):181‐8. - PubMed
TSA 2011 [Computer program]
-
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Turner 2012
van Valkenhoef 2012
-
- Valkenhoef G, Lu G, Brock B, Hillege H, Ades AE, Welton NJ. Automating network meta‐analysis. Research Synthesis Methods 2012;3(4):285‐99. - PubMed
Ware 2014
-
- Ware JE. SF‐36® health survey update, 2014. www.sf‐36.org/tools/sf36.shtml (accessed on 8 October 2014).
Wetterslev 2008
-
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. - PubMed
Wetterslev 2017
Wood 2008
Wright 1993
-
- Wright TL, Lau JY. Clinical aspects of hepatitis B virus infection. Lancet 1993;342(8883):1340‐4. - PubMed
Yang 2009
-
- Yang YF, Zhao W, Zhong YD, Xia HM, Shen L, Zhang N. Interferon therapy in chronic hepatitis B reduces progression to cirrhosis and hepatocellular carcinoma: a meta‐analysis. Journal of Viral Hepatitis 2009;16(4):265‐71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

