Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor
- PMID: 28322227
- PMCID: PMC5364407
- DOI: 10.1038/ncomms14902
Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor
Abstract
Reliable determination of binding kinetics and affinity of DNA hybridization and single-base mismatches plays an essential role in systems biology, personalized and precision medicine. The standard tools are optical-based sensors that are difficult to operate in low cost and to miniaturize for high-throughput measurement. Biosensors based on nanowire field-effect transistors have been developed, but reliable and cost-effective fabrication remains a challenge. Here, we demonstrate that a graphene single-crystal domain patterned into multiple channels can measure time- and concentration-dependent DNA hybridization kinetics and affinity reliably and sensitively, with a detection limit of 10 pM for DNA. It can distinguish single-base mutations quantitatively in real time. An analytical model is developed to estimate probe density, efficiency of hybridization and the maximum sensor response. The results suggest a promising future for cost-effective, high-throughput screening of drug candidates, genetic variations and disease biomarkers by using an integrated, miniaturized, all-electrical multiplexed, graphene-based DNA array.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


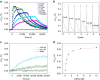
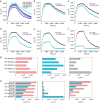
References
-
- Ahmed M. U., Saaem I., Wu P. C. & Brown A. S. Personalized diagnostics and biosensors: a review of the biology and technology needed for personalized medicine. Crit. Rev. Biotechnol. 34, 180–196 (2014). - PubMed
-
- Guo F. et al.. The Transcriptome and DNA methylome landscapes of human primordial germ cells. Cell 161, 1437–1452 (2015). - PubMed
-
- Ramsay G. et al.. DNA chips: state-of-the-art. Nat. Biotechnol. 16, 40–44 (1998). - PubMed
-
- Cooper M. A. et al.. Optical biosensors in drug discovery. Nat. Rev. Drug. Discov. 1, 515–528 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

