Effect of Dexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial
- PMID: 28322414
- PMCID: PMC5469298
- DOI: 10.1001/jama.2017.2088
Effect of Dexmedetomidine on Mortality and Ventilator-Free Days in Patients Requiring Mechanical Ventilation With Sepsis: A Randomized Clinical Trial
Abstract
Importance: Dexmedetomidine provides sedation for patients undergoing ventilation; however, its effects on mortality and ventilator-free days have not been well studied among patients with sepsis.
Objectives: To examine whether a sedation strategy with dexmedetomidine can improve clinical outcomes in patients with sepsis undergoing ventilation.
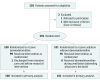
Design, setting, and participants: Open-label, multicenter randomized clinical trial conducted at 8 intensive care units in Japan from February 2013 until January 2016 among 201 consecutive adult patients with sepsis requiring mechanical ventilation for at least 24 hours.
Interventions: Patients were randomized to receive either sedation with dexmedetomidine (n = 100) or sedation without dexmedetomidine (control group; n = 101). Other agents used in both groups were fentanyl, propofol, and midazolam.
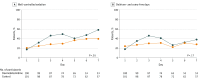
Main outcomes and measures: The co-primary outcomes were mortality and ventilator-free days (over a 28-day duration). Sequential Organ Failure Assessment score (days 1, 2, 4, 6, 8), sedation control, occurrence of delirium and coma, intensive care unit stay duration, renal function, inflammation, and nutrition state were assessed as secondary outcomes.
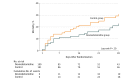
Results: Of the 203 screened patients, 201 were randomized. The mean age was 69 years (SD, 14 years); 63% were male. Mortality at 28 days was not significantly different in the dexmedetomidine group vs the control group (19 patients [22.8%] vs 28 patients [30.8%]; hazard ratio, 0.69; 95% CI, 0.38-1.22; P = .20). Ventilator-free days over 28 days were not significantly different between groups (dexmedetomidine group: median, 20 [interquartile range, 5-24] days; control group: median, 18 [interquartile range, 0.5-23] days; P = .20). The dexmedetomidine group had a significantly higher rate of well-controlled sedation during mechanical ventilation (range, 17%-58% vs 20%-39%; P = .01); other outcomes were not significantly different between groups. Adverse events occurred in 8 (8%) and 3 (3%) patients in the dexmedetomidine and control groups, respectively.
Conclusions and relevance: Among patients requiring mechanical ventilation, the use of dexmedetomidine compared with no dexmedetomidine did not result in statistically significant improvement in mortality or ventilator-free days. However, the study may have been underpowered for mortality, and additional research may be needed to evaluate this further.
Trial registration: clinicaltrials.gov Identifier: NCT01760967.
Conflict of interest statement
Figures
Comment in
-
Dexmedetomidine in Patients With Sepsis Requiring Mechanical Ventilation.JAMA. 2017 Aug 1;318(5):479-480. doi: 10.1001/jama.2017.7853. JAMA. 2017. PMID: 28763540 No abstract available.
-
Organ Function Assessment, Prevention, and Outcomes in Critically Ill Patients with Sepsis.Am J Respir Crit Care Med. 2018 Dec 1;198(11):1444-1446. doi: 10.1164/rccm.201705-0982RR. Am J Respir Crit Care Med. 2018. PMID: 30192644 No abstract available.
-
Trials of dexmedetomidine sedation in ventilated critically ill septic patients: Challenges, limitations and opportunities.Anaesth Crit Care Pain Med. 2021 Aug;40(4):100925. doi: 10.1016/j.accpm.2021.100925. Epub 2021 Jul 2. Anaesth Crit Care Pain Med. 2021. PMID: 34217839 No abstract available.
References
-
- Jakob SM, Ruokonen E, Grounds RM, et al. ; Dexmedetomidine for Long-Term Sedation Investigators . Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307(11):1151-1160. - PubMed
-
- Reade MC, Eastwood GM, Bellomo R, et al. ; DAHLIA Investigators; Australian and New Zealand Intensive Care Society Clinical Trials Group . Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA. 2016;315(14):1460-1468. - PubMed
-
- Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin-induced shock in rats. Crit Care Med. 2004;32(6):1322-1326. - PubMed
-
- Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose- and time-related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin-induced shock in rats. J Anesth. 2008;22(3):221-228. - PubMed
-
- Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17(5):576-584. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

