Antiviral Drug-Resistant Influenza B Viruses Carrying H134N Substitution in Neuraminidase, Laos, February 2016
- PMID: 28322707
- PMCID: PMC5367415
- DOI: 10.3201/eid2304.161876
Antiviral Drug-Resistant Influenza B Viruses Carrying H134N Substitution in Neuraminidase, Laos, February 2016
Abstract
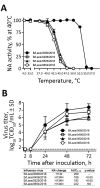
In February 2016, three influenza B/Victoria/2/87 lineage viruses exhibiting 4- to 158-fold reduced inhibition by neuraminidase inhibitors were detected in Laos. These viruses had an H134N substitution in the neuraminidase and replicated efficiently in vitro and in ferrets. Current antiviral drugs may be ineffective in controlling infections caused by viruses harboring this mutation.
Keywords: H134N substitution; Laos; antimicrobial resistance; antiviral drug–resistant; influenza; influenza B viruses; mutation; neuraminidase; viruses.
Figures
References
-
- Okomo-Adhiambo M, Mishin VP, Sleeman K, Saguar E, Guevara H, Reisdorf E, et al. Standardizing the influenza neuraminidase inhibition assay among United States public health laboratories conducting virological surveillance. Antiviral Res. 2016;128:28–35. 10.1016/j.antiviral.2016.01.009 - DOI - PubMed
-
- World Health Organization. Meetings of the WHO working group on surveillance of influenza antiviral susceptibility – Geneva, November 2011 and June 2012. Wkly Epidemiol Rec. 2012;87:369–74. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

