APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts
- PMID: 28323826
- PMCID: PMC5360223
- DOI: 10.1371/journal.pmed.1002254
APOE-related risk of mild cognitive impairment and dementia for prevention trials: An analysis of four cohorts
Abstract
Background: With the onset of prevention trials for individuals at high risk for Alzheimer disease, there is increasing need for accurate risk prediction to inform study design and enrollment, but available risk estimates are limited. We developed risk estimates for the incidence of mild cognitive impairment (MCI) or dementia among cognitively unimpaired individuals by APOE-e4 dose for the genetic disclosure process of the Alzheimer's Prevention Initiative Generation Study, a prevention trial in cognitively unimpaired APOE-e4/e4 homozygote individuals.
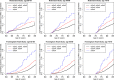
Methods and findings: We included cognitively unimpaired individuals aged 60-75 y, consistent with Generation Study eligibility criteria, from the National Alzheimer's Coordinating Center (NACC) (n = 5,073, 158 APOE-e4/e4), the Rotterdam Study (n = 6,399, 156 APOE-e4/e4), the Framingham Heart Study (n = 4,078, 67 APOE-e4/e4), and the Sacramento Area Latino Study on Aging (SALSA) (n = 1,294, 11 APOE-e4/e4). We computed stratified cumulative incidence curves by age (60-64, 65-69, 70-75 y) and APOE-e4 dose, adjusting for the competing risk of mortality, and determined risk of MCI and/or dementia by genotype and baseline age. We also used subdistribution hazard regression to model relative hazard based on age, APOE genotype, sex, education, family history of dementia, vascular risk, subjective memory concerns, and baseline cognitive performance. The four cohorts varied considerably in age, education, ethnicity/race, and APOE-e4 allele frequency. Overall, cumulative incidence was uniformly higher in NACC than in the population-based cohorts. Among APOE-e4/e4 individuals, 5-y cumulative incidence was as follows: in the 60-64-y age stratum, it ranged from 0% to 5.88% in the three population-based cohorts versus 23.06% in NACC; in the 65-69-y age stratum, from 9.42% to 10.39% versus 34.62%; and in the 70-75-y age stratum, from 18.64% to 33.33% versus 38.34%. Five-year incidence of dementia was negligible except for APOE-e4/e4 individuals and those over 70 y. Lifetime incidence (to age 80-85 y) of MCI or dementia for the APOE-e4/e4 individuals in the long-term Framingham and Rotterdam cohorts was 34.69%-38.45% at age 60-64 y, 30.76%-40.26% at 65-69 y, and 33.3%-35.17% at 70-75 y. Confidence limits for these estimates are often wide, particularly for APOE-e4/e4 individuals and for the dementia outcome at 5 y. In regression models, APOE-e4 dose and age both consistently increased risk, as did lower education, subjective memory concerns, poorer baseline cognitive performance, and family history of dementia. We discuss several limitations of the study, including the small numbers of APOE-e4/e4 individuals, missing data and differential dropout, limited ethnic and racial diversity, and differences in definitions of exposure and outcome variables.
Conclusions: Estimates of the absolute risk of MCI or dementia, particularly over short time intervals, are sensitive to sampling and a variety of methodological factors. Nonetheless, such estimates were fairly consistent across the population-based cohorts, and lower than those from a convenience cohort and those estimated in prior studies-with implications for informed consent and design for clinical trials targeting high-risk individuals.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Alzheimer’s Disease International. World alzheimer report 2016. London: Alzheimer’s Disease International; 2016 [cited 2016 October 26]. Available from: http://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

