Pyrrolidine dithiocarbamate administered during ex-vivo lung perfusion promotes rehabilitation of injured donor rat lungs obtained after prolonged warm ischemia
- PMID: 28323904
- PMCID: PMC5360331
- DOI: 10.1371/journal.pone.0173916
Pyrrolidine dithiocarbamate administered during ex-vivo lung perfusion promotes rehabilitation of injured donor rat lungs obtained after prolonged warm ischemia
Abstract
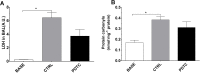
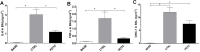
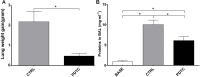
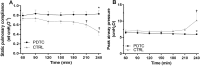
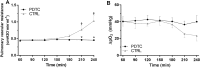
Damaged lung grafts obtained after circulatory death (DCD lungs) and warm ischemia may be at high risk of reperfusion injury after transplantation. Such lungs could be pharmacologically reconditioned using ex-vivo lung perfusion (EVLP). Since acute inflammation related to the activation of nuclear factor kappaB (NF-κB) is instrumental in lung reperfusion injury, we hypothesized that DCD lungs might be treated during EVLP by pyrrolidine dithiocarbamate (PDTC), an inhibitor of NF-κB. Rat lungs exposed to 1h warm ischemia and 2 h cold ischemia were subjected to EVLP during 4h, in absence (CTRL group, N = 6) or in presence of PDTC (2.5g/L, PDTC group, N = 6). Static pulmonary compliance (SPC), peak airway pressure (PAWP), pulmonary vascular resistance (PVR), and oxygenation capacity were determined during EVLP. After EVLP, we measured the weight gain of the heart-lung block (edema), and the concentration of LDH (cell damage), proteins (permeability edema) and of the cytokines IL-6, TNF-α and CINC-1 in bronchoalveolar lavage (BAL), and we evaluated NF-κB activation by the degree of phosphorylation and degradation of its inhibitor IκBα in lung tissue. In CTRL, we found significant NF-κB activation, lung edema, and a massive release of LDH, proteins and cytokines. SPC significantly decreased, PAWP and PVR increased, while oxygenation tended to decrease. Treatment with PDTC during EVLP inhibited NF-κB activation, did not influence LDH release, but markedly reduced lung edema and protein concentration in BAL, suppressed TNFα and IL-6 release, and abrogated the changes in SPC, PAWP and PVR, with unchanged oxygenation. In conclusion, suppression of innate immune activation during EVLP using the NF-κB inhibitor PDTC promotes significant improvement of damaged rat DCD lungs. Future studies will determine if such rehabilitated lungs are suitable for in vivo transplantation.
Conflict of interest statement
Figures


Similar articles
-
Postmortem and ex vivo carbon monoxide ventilation reduces injury in rat lungs transplanted from non-heart-beating donors.J Thorac Cardiovasc Surg. 2013 Aug;146(2):429-36.e1. doi: 10.1016/j.jtcvs.2012.11.005. Epub 2012 Dec 20. J Thorac Cardiovasc Surg. 2013. PMID: 23260460
-
Effects of cold or warm ischemia and ex-vivo lung perfusion on the release of damage associated molecular patterns and inflammatory cytokines in experimental lung transplantation.J Heart Lung Transplant. 2021 Sep;40(9):905-916. doi: 10.1016/j.healun.2021.05.015. Epub 2021 Jun 1. J Heart Lung Transplant. 2021. PMID: 34193360
-
Lungs donated after circulatory death and prolonged warm ischemia are transplanted successfully after enhanced ex vivo lung perfusion using adenosine A2B receptor antagonism.J Thorac Cardiovasc Surg. 2017 Nov;154(5):1811-1820. doi: 10.1016/j.jtcvs.2017.02.072. Epub 2017 Apr 12. J Thorac Cardiovasc Surg. 2017. PMID: 28483262 Free PMC article.
-
Ex vivo lung graft perfusion.Anaesth Crit Care Pain Med. 2016 Apr;35(2):123-31. doi: 10.1016/j.accpm.2015.09.006. Epub 2015 Dec 30. Anaesth Crit Care Pain Med. 2016. PMID: 26746565 Review.
-
Outcomes of marginal donors for lung transplantation after ex vivo lung perfusion: A systematic review and meta-analysis.J Thorac Cardiovasc Surg. 2020 Feb;159(2):720-730.e6. doi: 10.1016/j.jtcvs.2019.07.087. Epub 2019 Aug 25. J Thorac Cardiovasc Surg. 2020. PMID: 31548078
Cited by
-
The Versatility in the Applications of Dithiocarbamates.Int J Mol Sci. 2022 Jan 24;23(3):1317. doi: 10.3390/ijms23031317. Int J Mol Sci. 2022. PMID: 35163241 Free PMC article. Review.
-
Experimental Models of Ischemic Lung Damage for the Study of Therapeutic Reconditioning During Ex Vivo Lung Perfusion.Transplant Direct. 2022 Jun 10;8(7):e1337. doi: 10.1097/TXD.0000000000001337. eCollection 2022 Jul. Transplant Direct. 2022. PMID: 35702630 Free PMC article.
-
Transcriptomic Signatures in Lung Allografts and Their Therapeutic Implications.Biomedicines. 2024 Aug 7;12(8):1793. doi: 10.3390/biomedicines12081793. Biomedicines. 2024. PMID: 39200257 Free PMC article. Review.
-
Novel approaches for long-term lung transplant survival.Front Immunol. 2022 Jul 27;13:931251. doi: 10.3389/fimmu.2022.931251. eCollection 2022. Front Immunol. 2022. PMID: 35967365 Free PMC article. Review.
-
Cell type- and time-dependent biological responses in ex vivo perfused lung grafts.Front Immunol. 2023 Jul 3;14:1142228. doi: 10.3389/fimmu.2023.1142228. eCollection 2023. Front Immunol. 2023. PMID: 37465668 Free PMC article.
References
-
- Punch JD, Hayes DH, LaPorte FB, McBride V, Seely MS. Organ donation and utilization in the United States, 1996-2005. Am J Transplant. 2007;7(5 Pt 2):1327–38. - PubMed
-
- Steen S, Liao Q, Wierup PN, Bolys R, Pierre L, Sjoberg T. Transplantation of lungs from non-heart-beating donors after functional assessment ex vivo. Ann Thorac Surg. 2003;76(1):244–52. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

