β Cells Persist in T1D Pancreata Without Evidence of Ongoing β-Cell Turnover or Neogenesis
- PMID: 28323930
- PMCID: PMC5546851
- DOI: 10.1210/jc.2016-3806
β Cells Persist in T1D Pancreata Without Evidence of Ongoing β-Cell Turnover or Neogenesis
Abstract
Context: The cellular basis of persistent β-cell function in type 1 diabetes (T1D) remains enigmatic. No extensive quantitative β-cell studies of T1D pancreata have been performed to test for ongoing β-cell regeneration or neogenesis.
Objective: We sought to determine the mechanism of β-cell persistence in T1D pancreata.
Design: We studied T1D (n = 47) and nondiabetic control (n = 59) pancreata over a wide range of ages from the Juvenile Diabetes Research Foundation Network of Pancreatic Organ Donors with Diabetes via high-throughput microscopy.
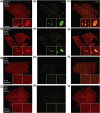
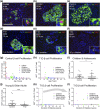
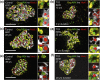
Intervention and main outcome measures: We quantified β-cell mass, β-cell turnover [via Ki-67 and terminal deoxynucleotide transferase-mediated dUTP nick end labeling (TUNEL)], islet ductal association, and insulin/glucagon coexpression in T1D and control pancreata.
Results: Residual insulin-producing β cells were detected in some (but not all) T1D cases of varying disease duration. Several T1D pancreata had substantial numbers of β cells. Although β-cell proliferation was prominent early in life, it dramatically declined after infancy in both nondiabetic controls and T1D individuals. However, β-cell proliferation was equivalent in control and T1D pancreata. β-cell death (assessed by TUNEL) was extremely rare in control and T1D pancreata. Thus, β-cell turnover was not increased in T1D. Furthermore, we found no evidence of small islet/ductal neogenesis or α-cell to β-cell transdifferentiation in T1D pancreata, regardless of disease duration.
Conclusion: Longstanding β-cell function in patients with T1D appears to be largely a result of β cells that persist, without any evidence of attempted β-cell regeneration, small islet/ductal neogenesis, or transdifferentiation from other islet endocrine cell types.
Copyright © 2017 by the Endocrine Society
Figures


References
-
- Gepts W. Pathologic anatomy of the pancreas in juvenile diabetes mellitus. Diabetes. 1965;14(10):619–633. - PubMed
-
- Gepts W, De Mey J. Islet cell survival determined by morphology. An immunocytochemical study of the islets of Langerhans in juvenile diabetes mellitus. Diabetes. 1978;27(Suppl 1):251–261. - PubMed
-
- Klöppel G, Löhr M, Habich K, Oberholzer M, Heitz PU. Islet pathology and the pathogenesis of type 1 and type 2 diabetes mellitus revisited. Surv Synth Pathol Res. 1985;4(2):110–125. - PubMed
-
- Butler AE, Galasso R, Meier JJ, Basu R, Rizza RA, Butler PC. Modestly increased beta cell apoptosis but no increased beta cell replication in recent-onset type 1 diabetic patients who died of diabetic ketoacidosis. Diabetologia. 2007;50(11):2323–2331. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

