Nonoperative Management or 'Watch and Wait' for Rectal Cancer with Complete Clinical Response After Neoadjuvant Chemoradiotherapy: A Critical Appraisal
- PMID: 28324284
- PMCID: PMC6106774
- DOI: 10.1245/s10434-017-5841-3
Nonoperative Management or 'Watch and Wait' for Rectal Cancer with Complete Clinical Response After Neoadjuvant Chemoradiotherapy: A Critical Appraisal
Abstract
Background: There is increasing interest in nonoperative management (NOM) for rectal cancer with complete clinical response (cCR) after neoadjuvant chemoradiation (nCRT).
Objective: The aim of this systematic review was to summarize the available data on NOM, with the intention of formulating standardized protocols on which to base future investigations.
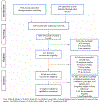
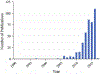
Methods: A systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines was conducted. A highly sensitive literature search identified all relevant studies published between January 2004 and December 2016. Data extraction and quality assessment was performed independently by two authors, and resolved by consensus with a third reviewer.
Results: In total, 15 studies, including 920 patients, met the inclusion criteria; 575 (62.5%) of these patients underwent NOM after cCR, with the remaining patients forming a surgical control group. The weighted mean follow-up was 39.4 (12.7) months in the NOM group and 39.8 (5.1) months in the surgery group. The pooled regrowth rate in the NOM group was 21.3% at a mean of 15.6 (7.0) months. Surgical salvage was possible and was undertaken in 93.2% of these patients. Overall survival in the NOM group was 91.7%, while disease-free survival was 82.7%. For the comparison proctectomy group, pooled rates of local recurrence, overall survival, and disease-free survival were 8.4, 92.4, and 87.5%, respectively.
Conclusion: NOM may be a feasible option for surgically eligible rectal cancer patients with cCR after nCRT. Before such a strategy can be widely implemented, further prospective data are required with standardized definitions, diagnostic criteria, and management protocols, with an emphasis on shared patient-provider decision making and patient-centered outcomes.
Conflict of interest statement
We declare that we have no relevant conflicts of interest.
Figures
References
-
- Tural D, Selcukbiricik F, Ozturk MA, et al. The relation between pathological complete response and clinical outcome in patients with rectal cancer. Hepato-gastroenterology. September 2013;60(126):1365–1370. - PubMed
-
- Perez RO. Complete clinical response in rectal cancer: a turning tide. The Lancet. Oncology. February 2016;17(2):125–126. - PubMed
-
- Hiotis SP, Weber SM, Cohen AM, et al. Assessing the predictive value of clinical complete response to neoadjuvant therapy for rectal cancer: an analysis of 488 patients. Journal of the American College of Surgeons. February 2002;194(2):131–135; discussion 135–136. - PubMed
-
- Neuman HB, Elkin EB, Guillem JG, et al. Treatment for patients with rectal cancer and a clinical complete response to neoadjuvant therapy: a decision analysis. Diseases of the colon and rectum. May 2009;52(5):863–871. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous