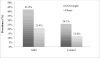Overweight and obese status in children with autism spectrum disorder and disruptive behavior
- PMID: 28325061
- PMCID: PMC5581311
- DOI: 10.1177/1362361316683888
Overweight and obese status in children with autism spectrum disorder and disruptive behavior
Abstract
Overweight and obesity are common in pediatric populations. Children with autism spectrum disorder and disruptive behavior may be at higher risk. This study examined whether children with autism spectrum disorder and disruptive behavior are more likely to be overweight or obese than matched controls. Baseline data from medication-free children with autism spectrum disorder who participated in trials conducted by the Research Units on Pediatric Psychopharmacology Autism Network (N = 276) were compared to 544 control children from the National Health and Nutrition Examination Survey database matched on age, sex, race, parent education, and era of data collection. The mean age of the children with autism spectrum disorder was 7.9 ± 2.6 years; 84.4% were males. In the autism spectrum disorder group, the prevalence was 42.4% for overweight and 21.4% for obesity compared to 26.1% for overweight and 12.0% for obesity among controls (p < 0.001 for each contrast). Within the autism spectrum disorder sample, obesity was associated with minority status and lower daily living skills. These findings suggest that children with autism spectrum disorder and disruptive behavior are at increased risk for obesity and underscore the need for weight management interventions in this population.
Keywords: National Health and Nutrition Examination Surveys; prevalence; risperidone.
Conflict of interest statement
Dr. Aman has received research contracts, consulted with, served on advisory boards, or done investigator training for CogState, Inc., CogState Clinical Trials, Ltd., Coronado Biosciences, Forest Research, Hoffman-La Roche, Lumos Pharma, MedAvante, Inc., ProPhase LLC, and Supernus Pharmaceuticals. Dr. J. McCracken has received NIMH research grant and contract funds; consultant income from Roche; research contract support from Seaside Pharmaceuticals and Roche; speaker honoraria from the Tourette Syndrome Association; and study drug and placebo from Shire. Dr. Arnold has received research funding from, Forest, Lilly, Shire, Supernus, and Young Living (as well as NIH and Autism Speaks) and has consulted with or been on advisory boards for Arbor, Otsuka, Pfizer, Roche, Seaside Therapeutics, and Waypoint. Dr. Vitiello has received salary support from NIH, and consultant fees from the American Physician Institute for Advanced Professional Studies. No other authors have potential conflicts of interest to disclose.
Figures
References
-
- Agranat-Meged AN, Deitcher C, Goldzweig G, et al. Childhood obesity and attention deficit/hyperactivity disorder: a newly described comorbidity in obese hospitalized children. The International Journal of Eating Disorders. 2005;37:357–359. - PubMed
-
- Aman MG, McDougle CJ, Scahill L, et al. Medication and parent training in children with pervasive developmental disorders and serious behavior problems: results from a randomized clinical trial. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48(12):1143–1154. - PMC - PubMed
-
- Aman MG, Singh NN, Stewart AW, et al. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. American Journal of Mental Deficiency. 1985;89(5):485–491. - PubMed
-
- Anderson SE, Cohen P, Naumova EN, et al. Relationship of childhood behavior disorders to weight gain from childhood into adulthood. Ambulatory Pediatrics. 2006;6:297–301. - PubMed
-
- Barlow SE, Expert Committee Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. - PubMed
Publication types
MeSH terms
Grants and funding
- M01 RR000034/RR/NCRR NIH HHS/United States
- U10 MH066764/MH/NIMH NIH HHS/United States
- U10 MH066768/MH/NIMH NIH HHS/United States
- U01 MH070009/MH/NIMH NIH HHS/United States
- U10 MH066766/MH/NIMH NIH HHS/United States
- M01 RR000750/RR/NCRR NIH HHS/United States
- M01 RR000052/RR/NCRR NIH HHS/United States
- U01 MH070010/MH/NIMH NIH HHS/United States
- K24 MH001805/MH/NIMH NIH HHS/United States
- M01 RR006022/RR/NCRR NIH HHS/United States
- K23 MH068627/MH/NIMH NIH HHS/United States
- N01 MH080011/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical