The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice
- PMID: 28325479
- PMCID: PMC5385998
- DOI: 10.1016/j.cmet.2017.02.008
The Role of Pancreatic Preproglucagon in Glucose Homeostasis in Mice
Abstract
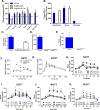
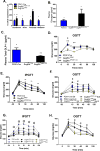
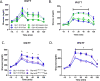
Glucagon-like peptide 1 (GLP-1) is necessary for normal gluco-regulation, and it has been widely presumed that this function reflects the actions of GLP-1 released from enteroendocrine L cells. To test the relative importance of intestinal versus pancreatic sources of GLP-1 for physiological regulation of glucose, we administered a GLP-1R antagonist, exendin-[9-39] (Ex9), to mice with tissue-specific reactivation of the preproglucagon gene (Gcg). Ex9 impaired glucose tolerance in wild-type mice but had no impact on Gcg-null or GLP-1R KO mice, suggesting that Ex9 is a true and specific GLP-1R antagonist. Unexpectedly, Ex-9 had no effect on blood glucose in mice with restoration of intestinal Gcg. In contrast, pancreatic reactivation of Gcg fully restored the effect of Ex9 to impair both oral and i.p. glucose tolerance. These findings suggest an alternative model whereby islet GLP-1 also plays an important role in regulating glucose homeostasis.
Keywords: GLP-1; glucose homeostasis; incretin.
Copyright © 2017 Elsevier Inc. All rights reserved.
Conflict of interest statement
DJD has served as an advisor or consultant to Arisaph Pharmaceuticals, Intarcia, Merck Research Laboratories, Novo Nordisk, and Receptors Inc and receives research funding from GSK, Merck, Novo Nordisk, and Sanofi Inc. RJS has received research support from Ethicon Endo-Surgery, Novo Nordisk, Sanofi, and Janssen. RJS has served on scientific advisory boards for, Ethicon Endo-Surgery, Daiichi Sankyo, Janssen, Novartis, Nestle, Takeda, Boehringer-Ingelheim, Sanofi, and Novo Nordisk. RJS is also a paid speaker for Ethicon Endo-Surgery. DAS has received research support from Ethicon Endo-Surgery, Novo Nordisk, and Boehringer Ingelheim.
Figures

Comment in
-
Diabetes: Pancreatic GLP1 is involved in glucose regulation.Nat Rev Endocrinol. 2017 May;13(5):252. doi: 10.1038/nrendo.2017.35. Epub 2017 Mar 24. Nat Rev Endocrinol. 2017. PMID: 28338662 No abstract available.
-
Pancreas and Not Gut Mediates the GLP-1-Induced Glucoincretin Effect.Cell Metab. 2017 Apr 4;25(4):757-758. doi: 10.1016/j.cmet.2017.03.020. Cell Metab. 2017. PMID: 28380367
-
Gut-Proglucagon-Derived Peptides Are Essential for Regulating Glucose Homeostasis in Mice.Cell Metab. 2019 Nov 5;30(5):976-986.e3. doi: 10.1016/j.cmet.2019.08.009. Epub 2019 Sep 5. Cell Metab. 2019. PMID: 31495689 Free PMC article.
References
-
- Ayala JE, Bracy DP, McGuinness OP, Wasserman DH. Considerations in the design of hyperinsulinemic-euglycemic clamps in the conscious mouse. Diabetes. 2006;55:390–397. - PubMed
-
- Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368:1696–1705. - PubMed
-
- Edwards CM, Todd JF, Mahmoudi M, Wang Z, Wang RM, Ghatei MA, Bloom SR. Glucagon-like peptide 1 has a physiological role in the control of postprandial glucose in humans: studies with the antagonist exendin 9–39. Diabetes. 1999;48:86–93. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

