DNase-active TREX1 frame-shift mutants induce serologic autoimmunity in mice
- PMID: 28325644
- PMCID: PMC5558601
- DOI: 10.1016/j.jaut.2017.03.001
DNase-active TREX1 frame-shift mutants induce serologic autoimmunity in mice
Abstract
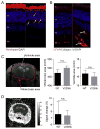
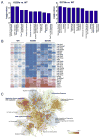
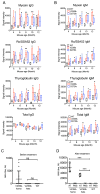
TREX1/DNASE III, the most abundant 3'-5' DNA exonuclease in mammalian cells, is tail-anchored on the endoplasmic reticulum (ER). Mutations at the N-terminus affecting TREX1 DNase activity are associated with autoimmune and inflammatory conditions such as Aicardi-Goutières syndrome (AGS). Mutations in the C-terminus of TREX1 cause loss of localization to the ER and dysregulation of oligosaccharyltransferase (OST) activity, and are associated with retinal vasculopathy with cerebral leukodystrophy (RVCL) and in some cases with systemic lupus erythematosus (SLE). Here we investigate mice with conditional expression of the most common RVCL mutation, V235fs, and another mouse expressing a conditional C-terminal mutation, D272fs, associated with a case of human SLE. Mice homozygous for either mutant allele express the encoded human TREX1 truncations without endogenous mouse TREX1, and both remain DNase active in tissues. The two mouse strains are similar phenotypically without major signs of retinal, cerebral or renal disease but exhibit striking elevations of autoantibodies in the serum. The broad range of autoantibodies is primarily against non-nuclear antigens, in sharp contrast to the predominantly DNA-related autoantibodies produced by a TREX1-D18N mouse that specifically lacks DNase activity. We also found that treatment with an OST inhibitor, aclacinomycin, rapidly suppressed autoantibody production in the TREX1 frame-shift mutant mice. Together, our study presents two new mouse models based on TREX1 frame-shift mutations with a unique set of serologic autoimmune-like phenotypes.
Copyright © 2017 Elsevier Ltd. All rights reserved.
Figures
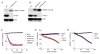



References
-
- Crow YJ, Manel N. Aicardi-Goutières syndrome and the type I interferonopathies. Nat Rev Immunol. 2015;15:429–440. http://dx.doi.org/10.1038/nri3850. - DOI - PubMed
-
- Richards A, van den Maagdenberg AMJM, Jen JC, Kavanagh D, Bertram P, Spitzer D, et al. C-terminal truncations in human 3′-5′ DNA exonuclease TREX1 cause autosomal dominant retinal vasculopathy with cerebral leukodystrophy. Nat Genet. 2007;39:1068–1070. http://dx.doi.org/10.1038/ng2082. - DOI - PubMed
-
- Stam AH, Kothari PH, Shaikh A, Gschwendter A, Jen JC, Hodgkinson S, et al. Retinal vasculopathy with cerebral leukoencephalopathy and systemic manifestations. Brain. 2016:aww217. http://dx.doi.org/10.1093/brain/aww217. - DOI - PMC - PubMed
-
- Stetson DB, Ko JS, Heidmann T, Medzhitov R. Trex1 prevents cell-intrinsic initiation of autoimmunity. Cell. 2008;134:587–598. http://dx.doi.org/10.1016/j.cell.2008.06.032. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

