Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection
- PMID: 28325763
- PMCID: PMC5362032
- DOI: 10.1128/mBio.00186-17
Pseudomonas aeruginosa Alginate Overproduction Promotes Coexistence with Staphylococcus aureus in a Model of Cystic Fibrosis Respiratory Infection
Abstract
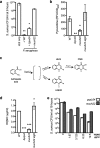
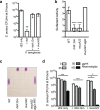
While complex intra- and interspecies microbial community dynamics are apparent during chronic infections and likely alter patient health outcomes, our understanding of these interactions is currently limited. For example, Pseudomonas aeruginosa and Staphylococcus aureus are often found to coinfect the lungs of patients with cystic fibrosis (CF), yet these organisms compete under laboratory conditions. Recent observations that coinfection correlates with decreased health outcomes necessitate we develop a greater understanding of these interbacterial interactions. In this study, we tested the hypothesis that P. aeruginosa and/or S. aureus adopts phenotypes that allow coexistence during infection. We compared competitive interactions of P. aeruginosa and S. aureus isolates from mono- or coinfected CF patients employing in vitro coculture models. P. aeruginosa isolates from monoinfected patients were more competitive toward S. aureus than P. aeruginosa isolates from coinfected patients. We also observed that the least competitive P. aeruginosa isolates possessed a mucoid phenotype. Mucoidy occurs upon constitutive activation of the sigma factor AlgT/U, which regulates synthesis of the polysaccharide alginate and dozens of other secreted factors, including some previously described to kill S. aureus Here, we show that production of alginate in mucoid strains is sufficient to inhibit anti-S. aureus activity independent of activation of the AlgT regulon. Alginate reduces production of siderophores, 2-heptyl-4-hydroxyquinolone-N-oxide (HQNO), and rhamnolipids-each required for efficient killing of S. aureus These studies demonstrate alginate overproduction may be an important factor driving P. aeruginosa coinfection with S. aureusIMPORTANCE Numerous deep-sequencing studies have revealed the microbial communities present during respiratory infections in cystic fibrosis (CF) patients are diverse, complex, and dynamic. We now face the challenge of determining the influence of these community dynamics on patient health outcomes and identifying candidate targets to modulate these interactions. We make progress toward this goal by determining that the polysaccharide alginate produced by mucoid strains of P. aeruginosa is sufficient to inhibit multiple secreted antimicrobial agents produced by this organism. Importantly, these secreted factors are required to outcompete S. aureus, when the microbes are grown in coculture; thus we propose a mechanism whereby mucoid P. aeruginosa can coexist with S. aureus Finally, the approach used here can serve as a platform to investigate the interactions among other CF pathogens.
Keywords: Pseudomonas aeruginosa; Staphylococcus aureus; biofilm; cystic fibrosis; mucoid; polymicrobial.
Copyright © 2017 Limoli et al.
Figures

Comment in
-
Evolution of Bacterial "Frenemies".mBio. 2017 May 23;8(3):e00675-17. doi: 10.1128/mBio.00675-17. mBio. 2017. PMID: 28536291 Free PMC article.
Similar articles
-
Exogenous Alginate Protects Staphylococcus aureus from Killing by Pseudomonas aeruginosa.J Bacteriol. 2020 Mar 26;202(8):e00559-19. doi: 10.1128/JB.00559-19. Print 2020 Mar 26. J Bacteriol. 2020. PMID: 31792010 Free PMC article.
-
Genotypic and Phenotypic Diversity of Staphylococcus aureus Isolates from Cystic Fibrosis Patient Lung Infections and Their Interactions with Pseudomonas aeruginosa.mBio. 2020 Jun 23;11(3):e00735-20. doi: 10.1128/mBio.00735-20. mBio. 2020. PMID: 32576671 Free PMC article.
-
Staphylococcus aureus and Pseudomonas aeruginosa Isolates from the Same Cystic Fibrosis Respiratory Sample Coexist in Coculture.Microbiol Spectr. 2022 Aug 31;10(4):e0097622. doi: 10.1128/spectrum.00976-22. Epub 2022 Jul 18. Microbiol Spectr. 2022. PMID: 35867391 Free PMC article.
-
Alginate production by the mucoid Pseudomonas aeruginosa associated with cystic fibrosis.Microbiol Sci. 1987 Oct;4(10):296-9. Microbiol Sci. 1987. PMID: 3155272 Review.
-
Help, hinder, hide and harm: what can we learn from the interactions between Pseudomonas aeruginosa and Staphylococcus aureus during respiratory infections?Thorax. 2019 Jul;74(7):684-692. doi: 10.1136/thoraxjnl-2018-212616. Epub 2019 Feb 18. Thorax. 2019. PMID: 30777898 Free PMC article. Review.
Cited by
-
Mucoid Pseudomonas aeruginosa Can Produce Calcium-Gelled Biofilms Independent of the Matrix Components Psl and CdrA.J Bacteriol. 2022 May 17;204(5):e0056821. doi: 10.1128/jb.00568-21. Epub 2022 Apr 13. J Bacteriol. 2022. PMID: 35416688 Free PMC article.
-
Surface-Enhanced Raman Scattering Spectroscopy for Label-Free Analysis of P. aeruginosa Quorum Sensing.Front Cell Infect Microbiol. 2018 May 11;8:143. doi: 10.3389/fcimb.2018.00143. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29868499 Free PMC article. Review.
-
Pseudomonas aeruginosa Biofilms.Int J Mol Sci. 2020 Nov 17;21(22):8671. doi: 10.3390/ijms21228671. Int J Mol Sci. 2020. PMID: 33212950 Free PMC article. Review.
-
The Impact of Intraspecies and Interspecies Bacterial Interactions on Disease Outcome.Pathogens. 2021 Jan 21;10(2):96. doi: 10.3390/pathogens10020096. Pathogens. 2021. PMID: 33494265 Free PMC article. Review.
-
Alginate exopolymer significantly modulates the viscoelastic properties and resilience of bacterial biofilms.NPJ Biofilms Microbiomes. 2025 Jun 9;11(1):98. doi: 10.1038/s41522-025-00718-6. NPJ Biofilms Microbiomes. 2025. PMID: 40490445 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

