Staphylococcal β-Toxin Modulates Human Aortic Endothelial Cell and Platelet Function through Sphingomyelinase and Biofilm Ligase Activities
- PMID: 28325766
- PMCID: PMC5362035
- DOI: 10.1128/mBio.00273-17
Staphylococcal β-Toxin Modulates Human Aortic Endothelial Cell and Platelet Function through Sphingomyelinase and Biofilm Ligase Activities
Abstract
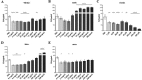
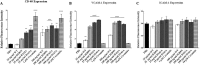
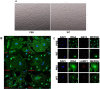
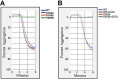
Staphylococcus aureus causes many infections, such as skin and soft tissue, pneumonia, osteomyelitis, and infective endocarditis (IE). IE is an endovascular infection of native and prosthetic valves and the lining of the heart; it is characterized by the formation of cauliflower-like "vegetations" composed of fibrin, platelets, other host factors, bacteria, and bacterial products. β-Toxin is an S. aureus virulence factor that contributes to the microorganism's ability to cause IE. This cytolysin has two enzymatic activities: sphingomyelinase (SMase) and biofilm ligase. Although both activities have functions in a rabbit model of IE, the mechanism(s) by which β-toxin directly affects human cells and is involved in the infectious process has not been elucidated. Here, we compared the in vitro effects of purified recombinant wild-type β-toxin, SMase-deficient β-toxin (H289N), and biofilm ligase-deficient β-toxin (H162A and/or D163A) on human aortic endothelial cells (HAECs) and platelets. β-Toxin was cytotoxic to HAECs and inhibited the production of interleukin 8 (IL-8) from these cells by both SMase and biofilm ligase activities. β-Toxin altered HAEC surface expression of CD40 and vascular cell adhesion molecule 1 (VCAM-1). HAECs treated with β-toxin displayed granular membrane morphology not seen in treatment with the SMase-deficient mutant. The altered morphology resulted in two possibly separable activities, cell rounding and redistribution of cell membranes into granules, which were not the result of endosome production from the Golgi apparatus or lysosomes. β-Toxin directly aggregated rabbit platelets via SMase activity.IMPORTANCE Each year there are up to 100,000 cases of infective endocarditis (IE) in the United States. S. aureus is the most common pathogen in patients with health care-associated IE and the leading cause of community-associated IE in the developed world. Multiple clonal group strains as defined by the Centers for Disease Control and Prevention, particularly USA200 and other clones encoding β-toxin, are highly associated with IE. Considering the strong association and established contribution of β-toxin in animal models of IE, determining how β-toxin directly affects human cell types, including endothelial cells and platelets, is important. In this study, we demonstrate that β-toxin functions to modulate endothelial cells and platelets by both toxin sphingomyelinase and biofilm ligase activities. Our data suggest that these activities modulate inflammation and increase infection severity.
Keywords: Staphylococcus aureus; beta-toxin; biofilm ligase; endothelial cells; sphingomyelinase.
Copyright © 2017 Herrera et al.
Figures

Similar articles
-
Staphylococcus aureus β-Toxin Mutants Are Defective in Biofilm Ligase and Sphingomyelinase Activity, and Causation of Infective Endocarditis and Sepsis.Biochemistry. 2016 May 3;55(17):2510-7. doi: 10.1021/acs.biochem.6b00083. Epub 2016 Apr 15. Biochemistry. 2016. PMID: 27015018 Free PMC article.
-
Staphylococcus aureus β-toxin production is common in strains with the β-toxin gene inactivated by bacteriophage.J Infect Dis. 2014 Sep 1;210(5):784-92. doi: 10.1093/infdis/jiu146. Epub 2014 Mar 11. J Infect Dis. 2014. PMID: 24620023 Free PMC article.
-
Beta toxin catalyzes formation of nucleoprotein matrix in staphylococcal biofilms.Proc Natl Acad Sci U S A. 2010 Aug 10;107(32):14407-12. doi: 10.1073/pnas.0911032107. Epub 2010 Jul 26. Proc Natl Acad Sci U S A. 2010. PMID: 20660751 Free PMC article.
-
Strategies for Survival of Staphylococcus aureus in Host Cells.Int J Mol Sci. 2025 Jan 16;26(2):720. doi: 10.3390/ijms26020720. Int J Mol Sci. 2025. PMID: 39859434 Free PMC article. Review.
-
[Interaction of Staphylococcus aureus alpha-toxin with eukaryotic cells and their target].Zh Mikrobiol Epidemiol Immunobiol. 2006 Mar-Apr;(2):110-4. Zh Mikrobiol Epidemiol Immunobiol. 2006. PMID: 16758912 Review. Russian.
Cited by
-
Comparative Genomic Reveals Clonal Heterogeneity in Persistent Staphylococcus aureus Infection.Front Cell Infect Microbiol. 2022 Feb 21;12:817841. doi: 10.3389/fcimb.2022.817841. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35265532 Free PMC article.
-
Computational insight into the protective mechanism of Allium iranicum Wendelbo. Alliaceae in a mouse model of Staphylococcosis: focus on dietary phytocannabinoid trans-caryophyllene.In Silico Pharmacol. 2021 Feb 7;9(1):17. doi: 10.1007/s40203-021-00078-x. eCollection 2021. In Silico Pharmacol. 2021. PMID: 33604234 Free PMC article.
-
An In Vitro Study of the Effect of Viburnum opulus Extracts on Key Processes in the Development of Staphylococcal Infections.Molecules. 2021 Mar 21;26(6):1758. doi: 10.3390/molecules26061758. Molecules. 2021. PMID: 33801012 Free PMC article.
-
Staphylococcus aureus β-Hemolysin Up-Regulates the Expression of IFN-γ by Human CD56bright NK Cells.Front Cell Infect Microbiol. 2021 Mar 29;11:658141. doi: 10.3389/fcimb.2021.658141. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 33854984 Free PMC article.
-
Oral hygiene might prevent cancer.Heliyon. 2018 Nov 2;4(10):e00879. doi: 10.1016/j.heliyon.2018.e00879. eCollection 2018 Oct. Heliyon. 2018. PMID: 30417145 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

