20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension
- PMID: 28325781
- PMCID: PMC5446268
- DOI: 10.1161/CIRCRESAHA.116.310525
20-HETE Signals Through G-Protein-Coupled Receptor GPR75 (Gq) to Affect Vascular Function and Trigger Hypertension
Abstract
Rationale: 20-Hydroxyeicosatetraenoic acid (20-HETE), one of the principle cytochrome P450 eicosanoids, is a potent vasoactive lipid whose vascular effects include stimulation of smooth muscle contractility, migration, and proliferation, as well as endothelial cell dysfunction and inflammation. Increased levels of 20-HETE in experimental animals and in humans are associated with hypertension, stroke, myocardial infarction, and vascular diseases.
Objective: To date, a receptor/binding site for 20-HETE has been implicated based on the use of specific agonists and antagonists. The present study was undertaken to identify a receptor to which 20-HETE binds and through which it activates a signaling cascade that culminates in many of the functional outcomes attributed to 20-HETE in vitro and in vivo.
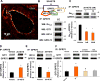
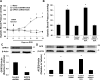
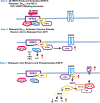
Methods and results: Using crosslinking analogs, click chemistry, binding assays, and functional assays, we identified G-protein receptor 75 (GPR75), currently an orphan G-protein-coupled receptor (GPCR), as a specific target of 20-HETE. In cultured human endothelial cells, 20-HETE binding to GPR75 stimulated Gαq/11 protein dissociation and increased inositol phosphate accumulation and GPCR-kinase interacting protein-1-GPR75 binding, which further facilitated the c-Src-mediated transactivation of epidermal growth factor receptor. This results in downstream signaling pathways that induce angiotensin-converting enzyme expression and endothelial dysfunction. Knockdown of GPR75 or GPCR-kinase interacting protein-1 prevented 20-HETE-mediated endothelial growth factor receptor phosphorylation and angiotensin-converting enzyme induction. In vascular smooth muscle cells, GPR75-20-HETE pairing is associated with Gαq/11- and GPCR-kinase interacting protein-1-mediated protein kinase C-stimulated phosphorylation of MaxiKβ, linking GPR75 activation to 20-HETE-mediated vasoconstriction. GPR75 knockdown in a mouse model of 20-HETE-dependent hypertension prevented blood pressure elevation and 20-HETE-mediated increases in angiotensin-converting enzyme expression, endothelial dysfunction, smooth muscle contractility, and vascular remodeling.
Conclusions: This is the first report to identify a GPCR target for an eicosanoid of this class. The discovery of 20-HETE-GPR75 pairing presented here provides the molecular basis for the signaling and pathophysiological functions mediated by 20-HETE in hypertension and cardiovascular diseases.
Keywords: cardiovascular diseases; cytochrome P-450 enzyme system; hypertension; receptor, epidermal growth factor; vascular remodeling.
© 2017 American Heart Association, Inc.
Figures



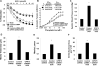

Comment in
-
GPR75 Identified as the First 20-HETE Receptor: A Chemokine Receptor Adopted by a New Family.Circ Res. 2017 May 26;120(11):1696-1698. doi: 10.1161/CIRCRESAHA.117.311022. Circ Res. 2017. PMID: 28546348 Free PMC article. No abstract available.
References
-
- Mancia G. Introduction to a compendium on hypertension. Circ Res. 2015;116:923–4. - PubMed
-
- Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6:731–736. - PubMed
-
- Roman RJ. P-450 metabolites of arachidonic Acid in the control of cardiovascular function. Physiological reviews. 2002;82:131–185. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

