A Specialized Diacylglycerol Acyltransferase Contributes to the Extreme Medium-Chain Fatty Acid Content of Cuphea Seed Oil
- PMID: 28325847
- PMCID: PMC5411140
- DOI: 10.1104/pp.16.01894
A Specialized Diacylglycerol Acyltransferase Contributes to the Extreme Medium-Chain Fatty Acid Content of Cuphea Seed Oil
Abstract
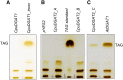
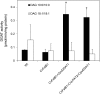
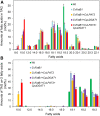
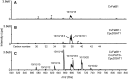
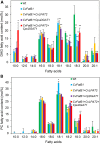
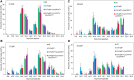
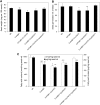
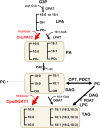
Seed oils of many Cuphea sp. contain >90% of medium-chain fatty acids, such as decanoic acid (10:0). These seed oils, which are among the most compositionally variant in the plant kingdom, arise from specialized fatty acid biosynthetic enzymes and specialized acyltransferases. These include lysophosphatidic acid acyltransferases (LPAT) and diacylglycerol acyltransferases (DGAT) that are required for successive acylation of medium-chain fatty acids in the sn-2 and sn-3 positions of seed triacylglycerols (TAGs). Here we report the identification of a cDNA for a DGAT1-type enzyme, designated CpuDGAT1, from the transcriptome of C. avigera var pulcherrima developing seeds. Microsomes of camelina (Camelina sativa) seeds engineered for CpuDGAT1 expression displayed DGAT activity with 10:0-CoA and the diacylglycerol didecanoyl, that was approximately 4-fold higher than that in camelina seed microsomes lacking CpuDGAT1. In addition, coexpression in camelina seeds of CpuDGAT1 with a C. viscosissima FatB thioesterase (CvFatB1) that generates 10:0 resulted in TAGs with nearly 15 mol % of 10:0. More strikingly, expression of CpuDGAT1 and CvFatB1 with the previously described CvLPAT2, a 10:0-CoA-specific Cuphea LPAT, increased 10:0 amounts to 25 mol % in camelina seed TAG. These TAGs contained up to 40 mol % 10:0 in the sn-2 position, nearly double the amounts obtained from coexpression of CvFatB1 and CvLPAT2 alone. Although enriched in diacylglycerol, 10:0 was not detected in phosphatidylcholine in these seeds. These findings are consistent with channeling of 10:0 into TAG through the combined activities of specialized LPAT and DGAT activities and demonstrate the biotechnological use of these enzymes to generate 10:0-rich seed oils.
© 2017 American Society of Plant Biologists. All Rights Reserved.
Figures


References
-
- Bafor M, Stymne S (1992) Substrate specificities of glycerol acylating enzymes from developing embryos of two Cuphea species. Phytochemistry 31: 2973–2976
-
- Bates PD. (2016) Understanding the control of acyl flux through the lipid metabolic network of plant oil biosynthesis. Biochim Biophys Acta 1861(9 Pt B): 1214–1225 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

