Perceptual integration without conscious access
- PMID: 28325878
- PMCID: PMC5389292
- DOI: 10.1073/pnas.1617268114
Perceptual integration without conscious access
Abstract
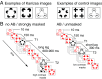
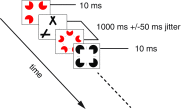
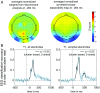
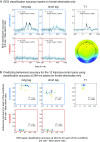
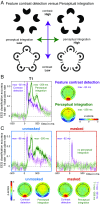
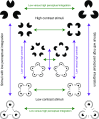
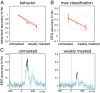
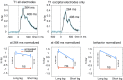
The visual system has the remarkable ability to integrate fragmentary visual input into a perceptually organized collection of surfaces and objects, a process we refer to as perceptual integration. Despite a long tradition of perception research, it is not known whether access to consciousness is required to complete perceptual integration. To investigate this question, we manipulated access to consciousness using the attentional blink. We show that, behaviorally, the attentional blink impairs conscious decisions about the presence of integrated surface structure from fragmented input. However, despite conscious access being impaired, the ability to decode the presence of integrated percepts remains intact, as shown through multivariate classification analyses of electroencephalogram (EEG) data. In contrast, when disrupting perception through masking, decisions about integrated percepts and decoding of integrated percepts are impaired in tandem, while leaving feedforward representations intact. Together, these data show that access consciousness and perceptual integration can be dissociated.
Keywords: access consciousness; attentional blink; masking; perceptual integration; phenomenal consciousness.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Helmholtz HV. Handbuch der Physiologischen Optik. Leopold Voss, Leipzig; Germany: 1867.
-
- Block N. Two neural correlates of consciousness. Trends Cogn Sci. 2005;9(2):46–52. - PubMed
-
- Lamme VAF. How neuroscience will change our view on consciousness. Cogn Neurosci. 2010;1(3):204–220. - PubMed
-
- Dehaene S, Changeux JP, Naccache L, Sackur J, Sergent C. Conscious, preconscious, and subliminal processing: A testable taxonomy. Trends Cogn Sci. 2006;10(5):204–211. - PubMed
-
- Baars BJ. Global workspace theory of consciousness: Toward a cognitive neuroscience of human experience. Prog Brain Res. 2005;150:45–53. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

