An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model
- PMID: 28325910
- PMCID: PMC5427874
- DOI: 10.1038/s41598-017-00193-w
An RNA nanoparticle vaccine against Zika virus elicits antibody and CD8+ T cell responses in a mouse model
Abstract
The Zika virus (ZIKV) outbreak in the Americas and South Pacific poses a significant burden on human health because of ZIKV's neurotropic effects in the course of fetal development. Vaccine candidates against ZIKV are coming online, but immunological tools to study anti-ZIKV responses in preclinical models, particularly T cell responses, remain sparse. We deployed RNA nanoparticle technology to create a vaccine candidate that elicited ZIKV E protein-specific IgG responses in C57BL/6 mice as assayed by ELISA. Using this tool, we identified a unique H-2Db-restricted epitope to which there was a CD8+ T cell response in mice immunized with our modified dendrimer-based RNA nanoparticle vaccine. These results demonstrate that this approach can be used to evaluate new candidate antigens and identify immune correlates without the use of live virus.
Conflict of interest statement
O.F.K., J.S.C., H.L.P., and D.G.A. have filed a patent on the MDNP-based vaccine platform.
Figures
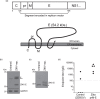
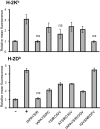
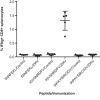
References
-
- Oehler, E. et al. Zika virus infection complicated by Guillain-Barre syndrome–case report, French Polynesia, December 2013. Euro Surveill19, doi:20720 (2014). - PubMed
-
- CDC. Zika virus disease in the United States, 2015–2016, http://www.cdc.gov/zika/geo/united-states.html (2016).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

