Systematic Review: FDA-Approved Prescription Medications for Adults With Constipation
- PMID: 28325992
- PMCID: PMC5358085
Systematic Review: FDA-Approved Prescription Medications for Adults With Constipation
Abstract
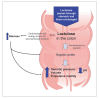
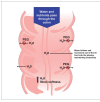
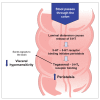
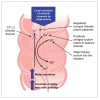
Constipation is a common, often chronic, gastrointestinal disorder that can negatively impact the lives of those it affects and can be difficult to treat satisfactorily. The objective of this systematic review is to identify and analyze the available published literature on US Food and Drug Administration-approved prescription therapies for adults with constipation (episodic and chronic) and to assess their place in therapy, based on the methodologic strength and results of identified clinical trials. Ovid MEDLINE, PubMed, and EMBASE databases were used to search the published literature. Studies were included if they were randomized and prospective, conducted in adults (age ≥18), published as full-length manuscripts in English, and compared the test agent with placebo or a comparator(s). Studies were excluded if they involved patients with constipation attributed to secondary causes. Because fully published manuscripts from phase III efficacy trials involving the recently approved medication lubiprostone were not available, a manual search was performed of abstracts from the two annual major gastroenterology meetings (American College of Gastroenterology and Digestive Disease Week) from the past 4 years. Data on study design; number, age, and sex of patients; duration of treatment period; primary efficacy variable; secondary efficacy variables; adverse events; and discontinuations because of adverse events were abstracted from eligible articles. Eligible studies were assessed using well-established recommendations and a preformatted standardized form. A scoring system, with scores ranging from 1 to 15, was used to individually and separately assess the methodologic quality of the studies. Results of this analysis indicate a general lack of methodologically high-quality clinical trials supporting the use of lactulose and PEG 3350 to treat patients with chronic constipation, but data support their use in acute, episodic constipation. Conversely, high-quality evidence for tegaserod and lubiprostone in patients with chronic constipation does exist, though conclusions regarding the role in therapy for lubiprostone are still in development.
Keywords: Constipation; PEG 3350; lactulose; lubiprostone; polyethylene glycol; tegaserod.
Figures
References
-
- Higgins PD, Johanson JF. Epidemiology of constipation in North America: a systematic review. Am J Gastroenterol. 2004;99:750–759. - PubMed
-
- Pare P, Ferrazzi S, Thompson WG, et al. An epidemiological survey of constipation in Canada: definitions, rates, demographics, and predictors of health care seeking. Am J Gastroenterol. 2001;96:3130–3137. - PubMed
-
- Irvine EJ, Ferrazzi S, Pare P, et al. Health-related quality of life in functional GI disorders: focus on constipation and resource utilization. Am J Gastroenterol. 2002;97:1986–1993. - PubMed
-
- Dennison C, Prasad M, Lloyd A, et al. The health-related quality of life and economic burden of constipation. PharmacoEconomics. 2005;23:461–476. - PubMed
-
- Harris LA. Prevalence and ramifications of chronic constipation. Manag Care Interface. 2005:23–30. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous