Molecular Chaperone Calnexin Regulates the Function of Drosophila Sodium Channel Paralytic
- PMID: 28326013
- PMCID: PMC5339336
- DOI: 10.3389/fnmol.2017.00057
Molecular Chaperone Calnexin Regulates the Function of Drosophila Sodium Channel Paralytic
Abstract
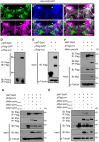
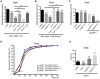
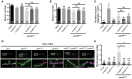
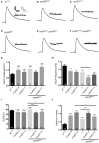
Neuronal activity mediated by voltage-gated channels provides the basis for higher-order behavioral tasks that orchestrate life. Chaperone-mediated regulation, one of the major means to control protein quality and function, is an essential route for controlling channel activity. Here we present evidence that Drosophila ER chaperone Calnexin colocalizes and interacts with the α subunit of sodium channel Paralytic. Co-immunoprecipitation analysis indicates that Calnexin interacts with Paralytic protein variants that contain glycosylation sites Asn313, 325, 343, 1463, and 1482. Downregulation of Calnexin expression results in a decrease in Paralytic protein levels, whereas overexpression of the Calnexin C-terminal calcium-binding domain triggers an increase reversely. Genetic analysis using adult climbing, seizure-induced paralysis, and neuromuscular junction indicates that lack of Calnexin expression enhances Paralytic-mediated locomotor deficits, suppresses Paralytic-mediated ghost bouton formation, and regulates minature excitatory junction potentials (mEJP) frequency and latency time. Taken together, our findings demonstrate a need for chaperone-mediated regulation on channel activity during locomotor control, providing the molecular basis for channlopathies such as epilepsy.
Keywords: Calnexin chaperone; NMJ synaptogenesis; locomotor behavior; paralytic; sodium channel.
Figures


Similar articles
-
Calnexin associates with Shaker K+ channel protein but is not involved in quality control of subunit folding or assembly.Recept Channels. 1999;6(4):229-39. Recept Channels. 1999. PMID: 10412717
-
The Lectin Chaperone Calnexin Is Involved in the Endoplasmic Reticulum Stress Response by Regulating Ca2+ Homeostasis in Aspergillus nidulans.Appl Environ Microbiol. 2017 Jul 17;83(15):e00673-17. doi: 10.1128/AEM.00673-17. Print 2017 Aug 1. Appl Environ Microbiol. 2017. PMID: 28550061 Free PMC article.
-
Calnexin co-expression and the use of weaker promoters increase the expression of correctly assembled Shaker potassium channel in insect cells.Biochim Biophys Acta. 2003 Feb 17;1610(1):124-32. doi: 10.1016/s0005-2736(02)00715-0. Biochim Biophys Acta. 2003. PMID: 12586386
-
The Merck Frosst Award Lecture 1994/La conference Merck Frosst 1994. Calnexin: a molecular chaperone with a taste for carbohydrate.Biochem Cell Biol. 1995 Mar-Apr;73(3-4):123-32. doi: 10.1139/o95-015. Biochem Cell Biol. 1995. PMID: 7576485 Review.
-
Calnexin: a membrane-bound chaperone of the endoplasmic reticulum.Trends Biochem Sci. 1994 Mar;19(3):124-8. doi: 10.1016/0968-0004(94)90205-4. Trends Biochem Sci. 1994. PMID: 8203019 Review.
Cited by
-
Can Plant Lectins Help to Elucidate Insect Lectin-Mediated Immune Response?Insects. 2021 May 27;12(6):497. doi: 10.3390/insects12060497. Insects. 2021. PMID: 34071763 Free PMC article. Review.
-
Drosophila Voltage-Gated Sodium Channels Are Only Expressed in Active Neurons and Are Localized to Distal Axonal Initial Segment-like Domains.J Neurosci. 2020 Oct 14;40(42):7999-8024. doi: 10.1523/JNEUROSCI.0142-20.2020. Epub 2020 Sep 14. J Neurosci. 2020. PMID: 32928889 Free PMC article.
-
FoxO suppresses endoplasmic reticulum stress to inhibit growth of Tsc1-deficient tissues under nutrient restriction.Elife. 2020 Jun 11;9:e53159. doi: 10.7554/eLife.53159. Elife. 2020. PMID: 32525804 Free PMC article.
-
The Tenets of Teneurin: Conserved Mechanisms Regulate Diverse Developmental Processes in the Drosophila Nervous System.Front Neurosci. 2019 Jan 30;13:27. doi: 10.3389/fnins.2019.00027. eCollection 2019. Front Neurosci. 2019. PMID: 30760977 Free PMC article.
-
6-Hydroxydopamine Induces Neurodegeneration in Terminally Differentiated SH-SY5Y Neuroblastoma Cells via Enrichment of the Nucleosomal Degradation Pathway: a Global Proteomics Approach.J Mol Neurosci. 2022 May;72(5):1026-1046. doi: 10.1007/s12031-021-01962-z. Epub 2022 Mar 8. J Mol Neurosci. 2022. PMID: 35258800 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

