Arabidopsis NAC Transcription Factor JUNGBRUNNEN1 Exerts Conserved Control Over Gibberellin and Brassinosteroid Metabolism and Signaling Genes in Tomato
- PMID: 28326087
- PMCID: PMC5339236
- DOI: 10.3389/fpls.2017.00214
Arabidopsis NAC Transcription Factor JUNGBRUNNEN1 Exerts Conserved Control Over Gibberellin and Brassinosteroid Metabolism and Signaling Genes in Tomato
Abstract
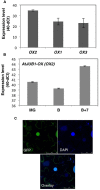
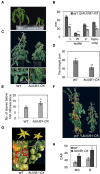
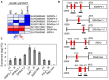
The Arabidopsis thaliana NAC transcription factor JUNGBRUNNEN1 (AtJUB1) regulates growth by directly repressing GA3ox1 and DWF4, two key genes involved in gibberellin (GA) and brassinosteroid (BR) biosynthesis, respectively, leading to GA and BR deficiency phenotypes. AtJUB1 also reduces the expression of PIF4, a bHLH transcription factor that positively controls cell elongation, while it stimulates the expression of DELLA genes, which are important repressors of growth. Here, we extend our previous findings by demonstrating that AtJUB1 induces similar GA and BR deficiency phenotypes and changes in gene expression when overexpressed in tomato (Solanum lycopersicum). Importantly, and in accordance with the growth phenotypes observed, AtJUB1 inhibits the expression of growth-supporting genes, namely the tomato orthologs of GA3ox1, DWF4 and PIF4, but activates the expression of DELLA orthologs, by directly binding to their promoters. Overexpression of AtJUB1 in tomato delays fruit ripening, which is accompanied by reduced expression of several ripening-related genes, and leads to an increase in the levels of various amino acids (mostly proline, β-alanine, and phenylalanine), γ-aminobutyric acid (GABA), and major organic acids including glutamic acid and aspartic acid. The fact that AtJUB1 exerts an inhibitory effect on the GA/BR biosynthesis and PIF4 genes but acts as a direct activator of DELLA genes in both, Arabidopsis and tomato, strongly supports the model that the molecular constituents of the JUNGBRUNNEN1 growth control module are considerably conserved across species.
Keywords: Arabidopsis; DELLA proteins; brassinosteroid; fruit; gibberellic acid; growth; tomato; transcription factor.
Figures


References
-
- Alexander L., Grierson D. (2002). Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening. J. Exp. Bot. 53 2039–2055. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

