(Z)-Selective Takai olefination of salicylaldehydes
- PMID: 28326141
- PMCID: PMC5331342
- DOI: 10.3762/bjoc.13.35
(Z)-Selective Takai olefination of salicylaldehydes
Abstract
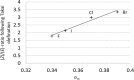
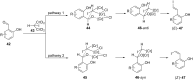
The Takai olefination (or Takai reaction) is a method for the conversion of aldehydes to vinyl iodides, and has seen widespread implementation in organic synthesis. The reaction is usually noted for its high (E)-selectivity; however, herein we report the highly (Z)-selective Takai olefination of salicylaldehyde derivatives. Systematic screening of related substrates led to the identification of key factors responsible for this surprising inversion of selectivity, and enabled the development of a modified mechanistic model to rationalise these observations.
Keywords: Takai olefination; alkenyl iodides; salicylaldehydes; stereoselectivity; transition state.
Figures

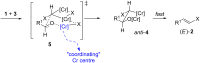



References
-
- Kürti L, Czakó B. Strategic Applications of Named Reactions in Organic Synthesis Background and Detailed Mechanisms. Oxford, U.K.: Elsevier Science; 2005.
-
- Hodgson D M. Tetrahedron Lett. 1992;33:5603–5604. doi: 10.1016/S0040-4039(00)61158-9. - DOI
-
- Takai K, Kataoka Y, Okazoe T, Utimoto K. Tetrahedron Lett. 1987;28:1443–1446. doi: 10.1016/S0040-4039(00)95949-5. - DOI
-
- Takai K, Shinomiya N, Kaihara H, Yoshida N, Moriwake T, Utimoto K. Synlett. 1995:963–964. doi: 10.1055/s-1995-5141. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
